“This product assures me of accuracy and with its ease of use, it is always ready to use. It doesn’t require reconstitution, and thus removes most operator error from determination.”
SelectScience Community Member review of A1c-Cellular Linearity
A1c-Cellular Linearity is a ready-to-use linearity product that tests the entire HbA1c procedure, including the lysing of the red blood cell. It is assayed on the top HbA1c chemistry analyzers and provides instrument-specific target values and expected ranges.
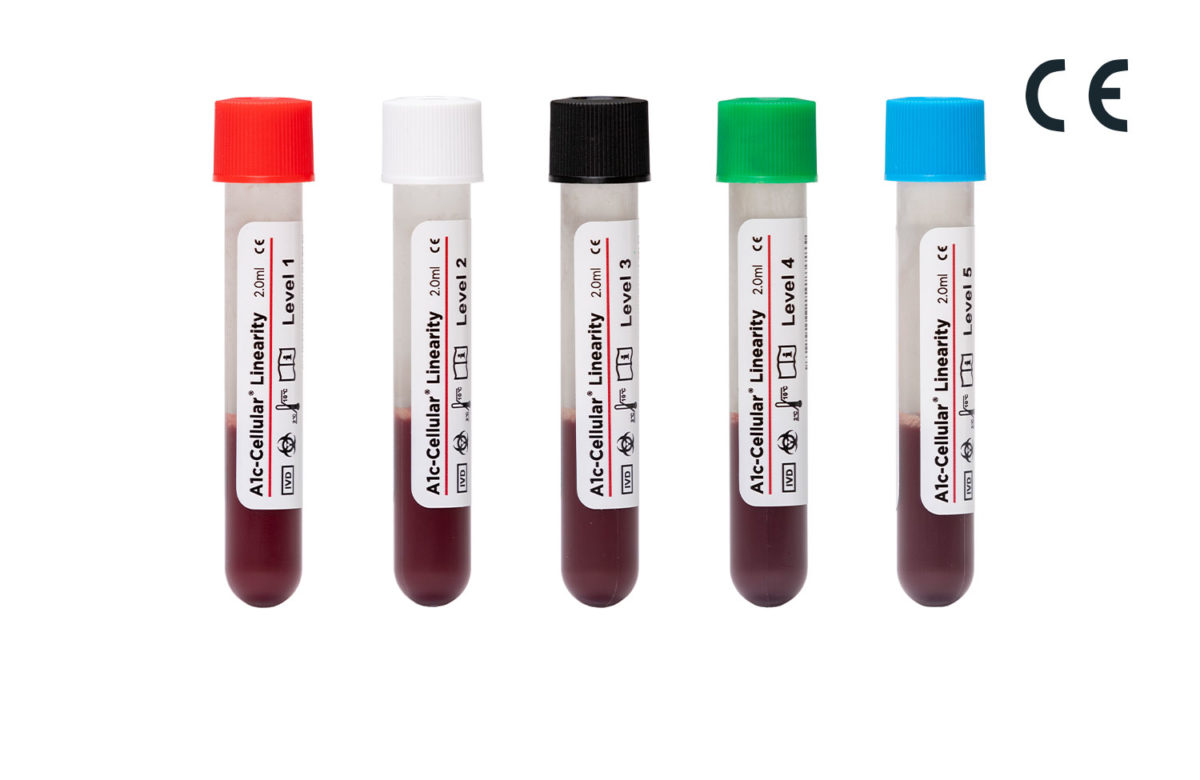
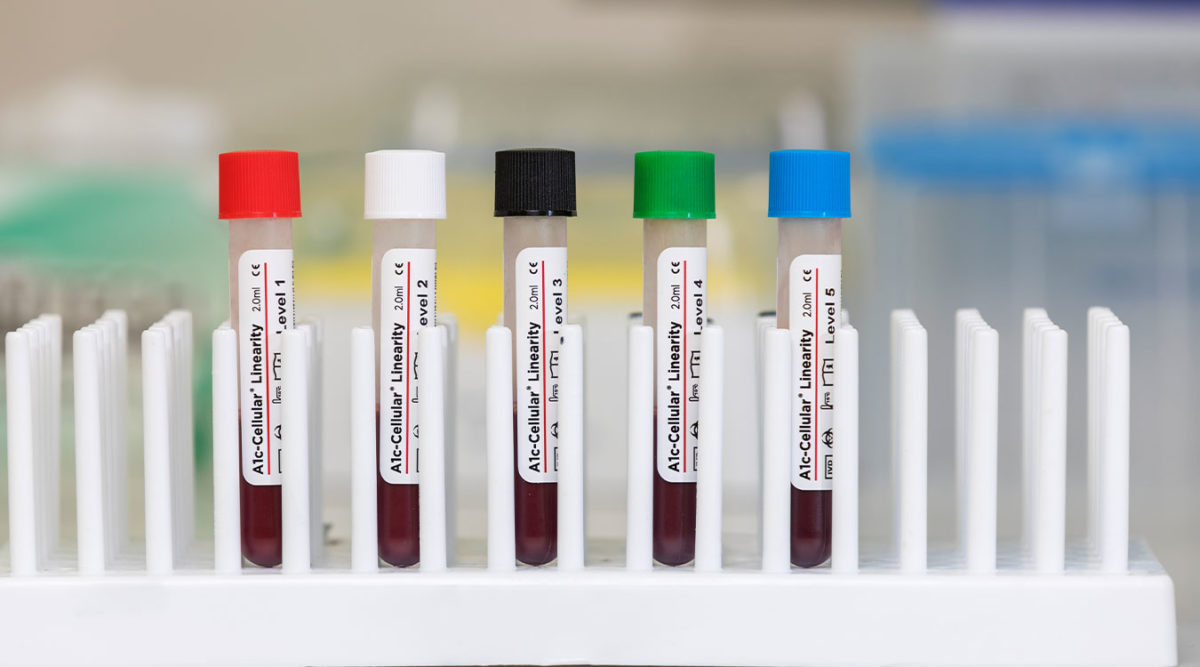
A1c-Cellular Linearity is a 5-level assayed linearity material used to assess instrument accuracy and verify the reportable range of the HbA1c parameter. Because A1c-Cellular Linearity contains intact red blood cells, it tests the entire HbA1c procedure including red blood cell lysis, a step that is not evaluated by competitor controls. A1c-Cellular Linearity does not need to be reconstituted prior to use, reducing the potential for human error, and is packaged in convenient vials with pierceable caps for autosampling and barcodes for instruments capable of barcode scanning…
| Description | Catalog Number |
|---|---|
| 5 x 2.0 mL | 285536 |
New customers
Existing customers
International customers find a distributor
Looking for an older document? Click here for archived assays and certificates.
Streck’s HbA1c product line includes a control and linearity with intact red blood cells to challenge the entire HbA1c procedure, including the lysing of the red blood cells. Daily use of these whole blood controls provides quality control data for confirming the precision and accuracy of your laboratory’s instruments. Laboratories should validate test methods at least every six months or following changes in lots of critical reagents or system components.