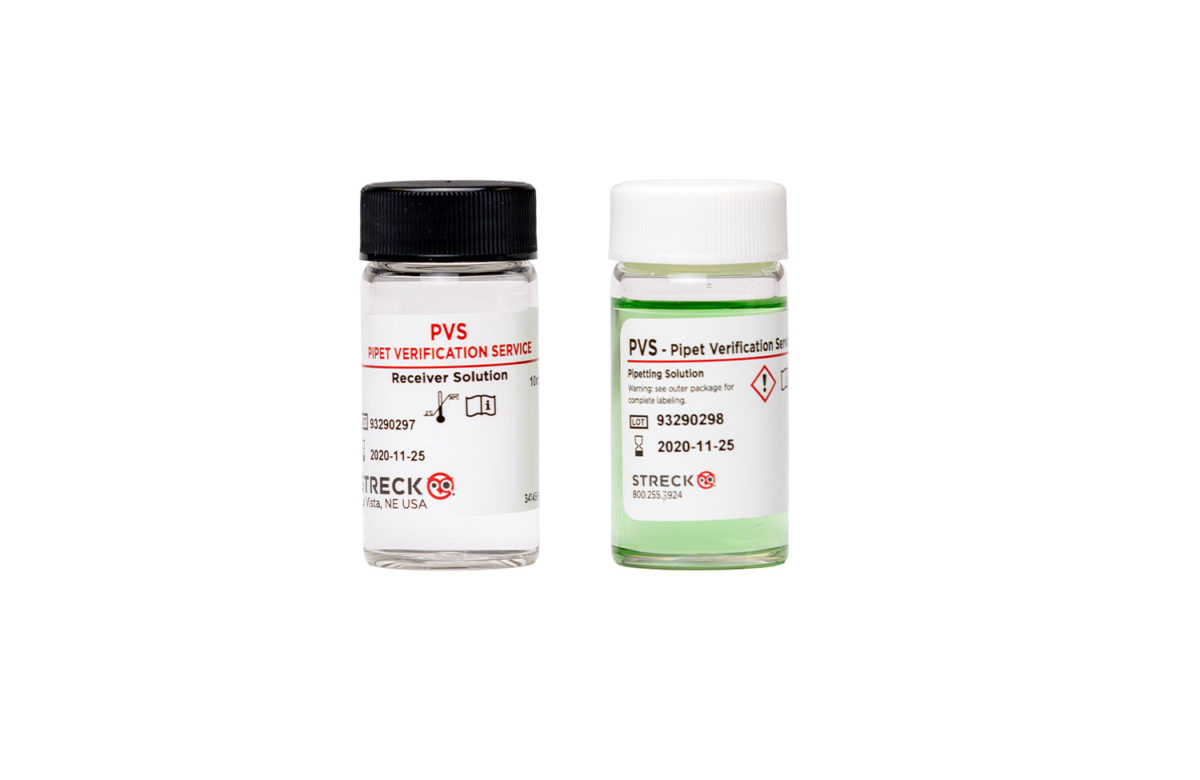
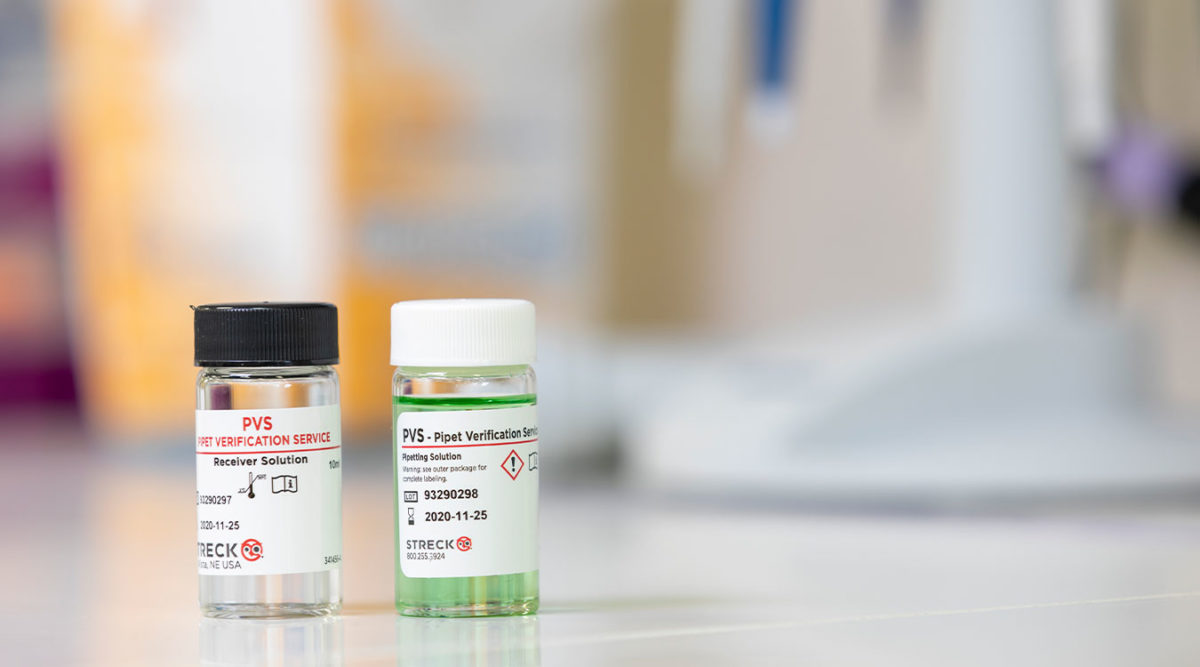
“Our lab has used the Pipet Verification Service for four years now and will continue in the future. We order the kits through our distributor; they are sent to us quickly and we use them when we have time set aside. I like that the pipets never leave the lab. The kit is easy to use and is sent to Streck through regular mail in a prepaid box. The results are sent via email on a very nice form with all the information needed. One of the easiest ways to achieve compliance.”
SelectScience Community Member review of Pipet Verification Service