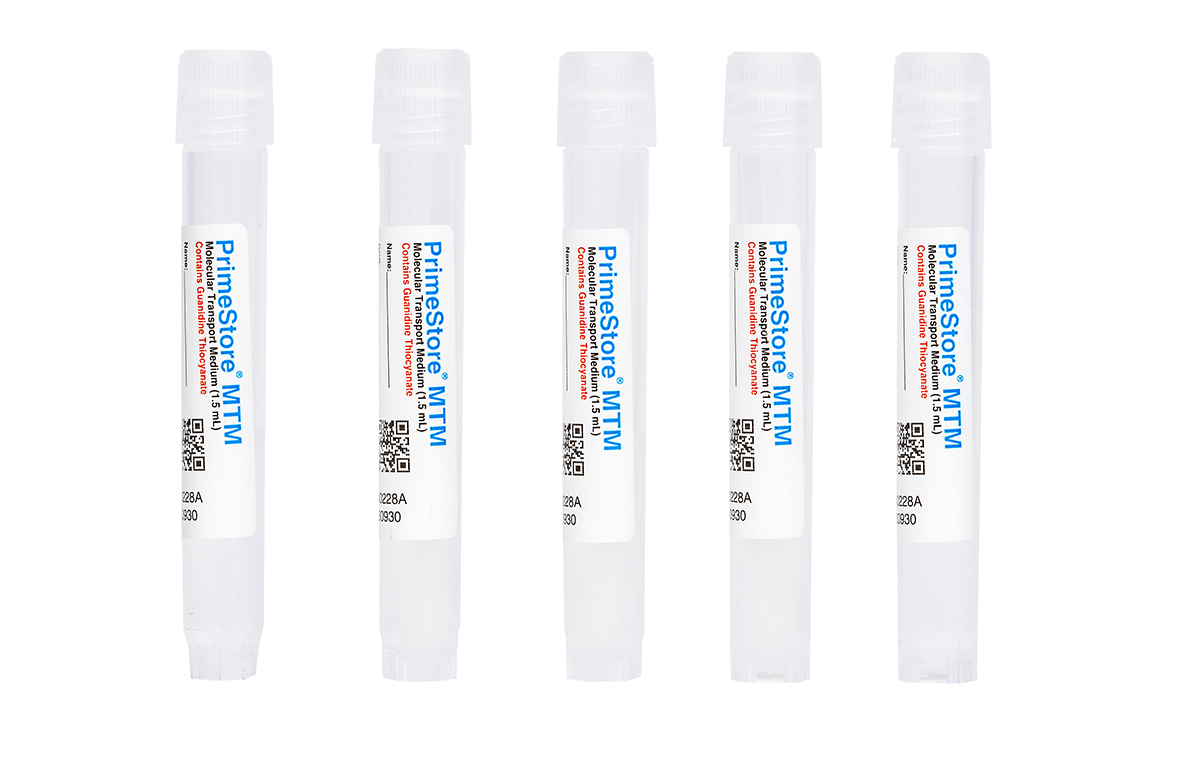
PrimeStore® MTM
PrimeStore MTM is designed to rapidly inactivate viruses (including Influenza), bacteria (including Mycobacterium tuberculosis, MTB) and veterinary pathogens (ASFv, CSFv, FMDv, HPAI, and NDv) at point-of-collection and stabilize DNA and RNA during storage and shipping. Samples can be collected, shipped and processed without prior inactivation and at ambient temperature.
Product Description
PrimeStore MTM is a cost-effective and safe option for the collection and transport of infectious disease samples for point-of-care and reference laboratories doing nucleic acid-based testing. It is formulated to:
- Rapidly inactivate potentially pathogenic microorganisms for safe sample handling.
- Preserve and stabilize nucleic acids (DNA, RNA, mRNA) at ambient temperatures.
- Be used with various nucleic acid extraction processes, point-of-care and syndromic testing platforms.

FDA Cleared Class II. For use in diagnostic procedures.
Features
- One solution for multiple sample types including blood/plasma/serum, body fluids (urine, sputum, saliva), swabs (oral, throat, nasal, nasopharyngeal, buccal) and more *
- Preserves sample-derived DNA, RNA and mRNA during extended ambient temperature storage
- Compatible with most nucleic acid extraction methods and high-throughput systems
* For additional sample examples, click here.
Benefits
- Rapid and consistent inactivation of pathogens means no requirement for BSL-2 facilities
- Extended sample stability eliminates the need for immediate processing
- Room temperature storage reduces costs and complications associated with cold chain shipping

Lab Notes
Learn how PrimeStore MTM helped Aegis® Sciences Corporation collect, inactivate, stabilize and preserve critical samples during the COVID-19 pandemic.
Ordering Information
| Description | Catalog Number |
|---|---|
| 50-tube pack (5.0 mL) | 230670 |
Resources
Instructions IFU
Certificates
Product Information
Looking for an older document? Click here for archived assays and certificates.
Molecular Products
With full-process controls and antibiotic resistance kits, Streck has the products to help you streamline your molecular diagnostics testing, aid antibiotic stewardship and ensure positive patient outcomes.
The latest from the blog
New antibiotic fights antimicrobial resistant bacteria