Body Fluids
Have confidence in your body fluid counts.
With our line of manual and automated body fluid and sperm count controls, you can be certain in your body fluid testing methods and results.
Streck customers also have access to STATS®, our free interlaboratory quality control program with peer group data comparison and online submission.
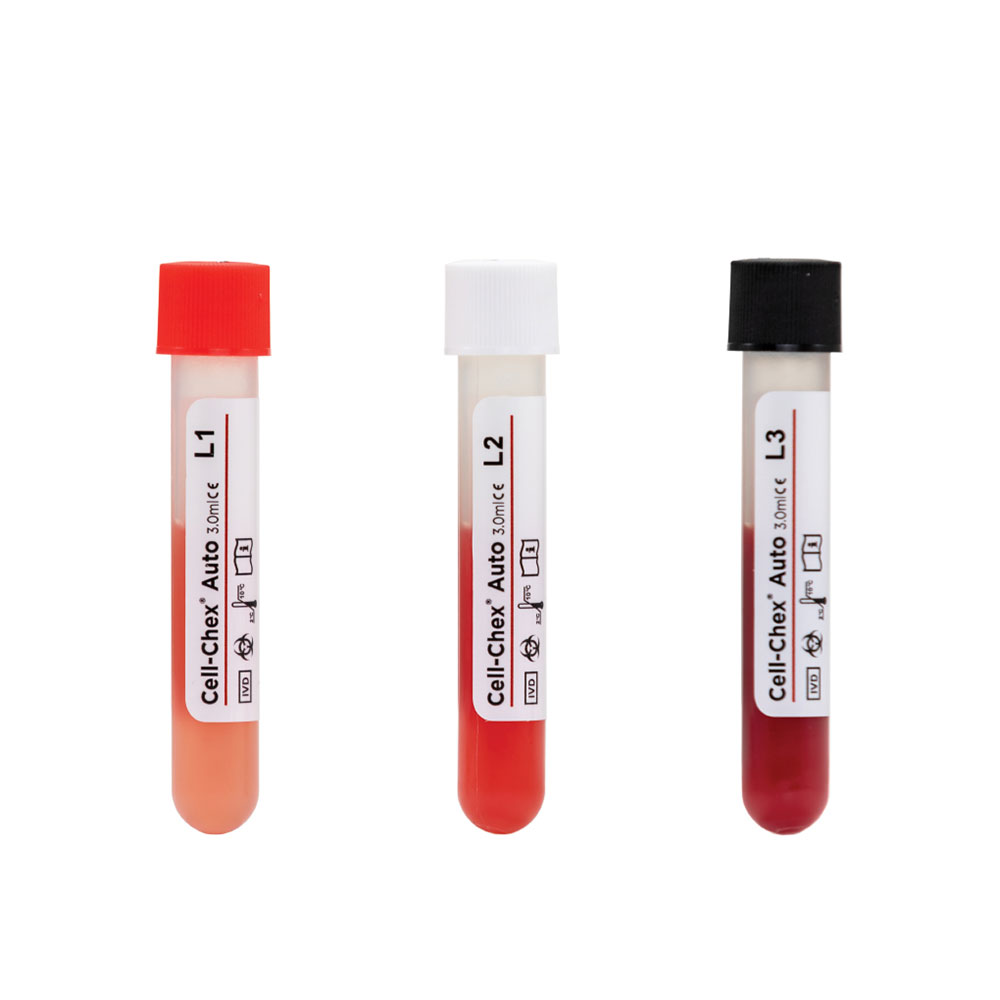
Cell-Chex®
A multi-level manual cerebrospinal and body fluid control for evaluating the accuracy and precision of hemacytometer RBC and WBC counts, urate and CPPD crystal identification and manual…
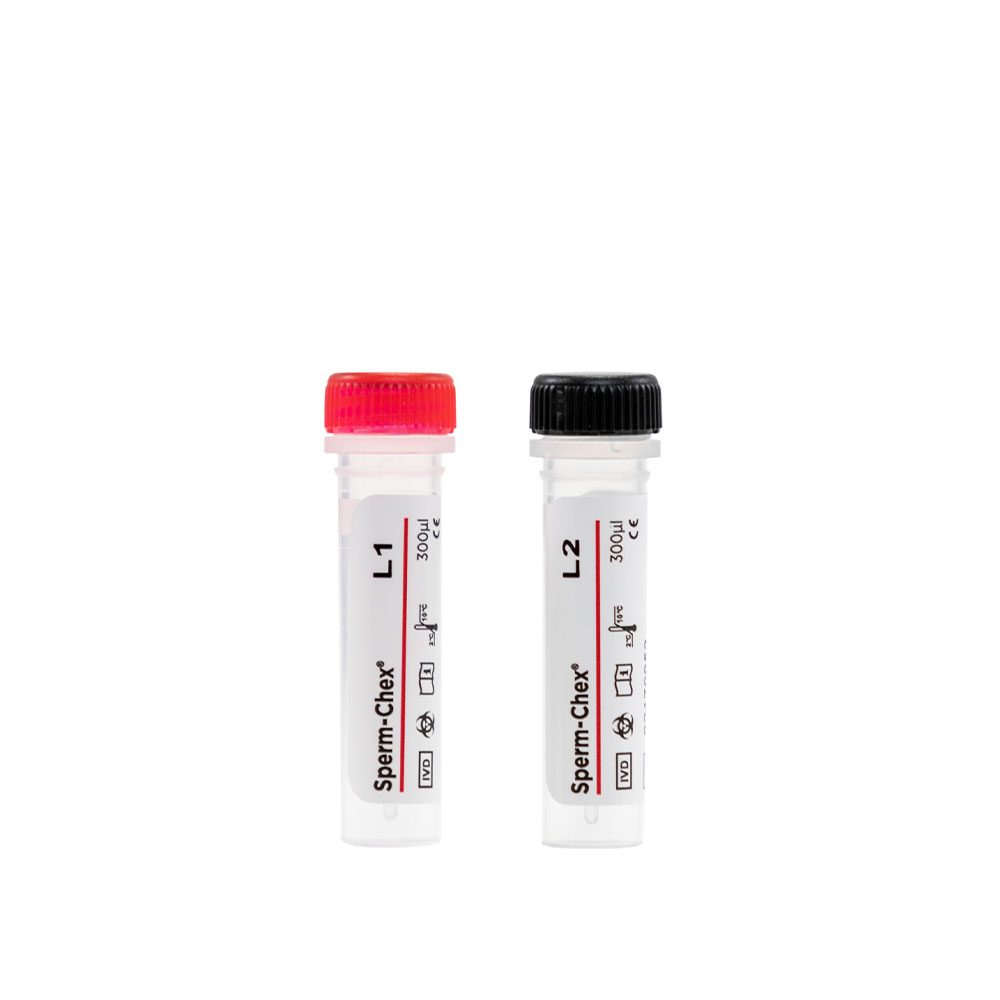
Sperm-Chex®
A sperm count control that contains real stabilized sperm cells in two clinically significant levels to validate the accuracy and precision of your laboratory’s manual sperm counting methods….
Sperm-Chex® Post VC
A post-vasectomy sperm count control that validates the manual post-vasectomy sperm count procedure. Comprised of real stabilized sperm cells, Sperm-Chex Post VC can be handled like a patient…
The latest from the blog

CHMP positive about Pfizer’s new combination antibiotic