The Benefits of Glass BCTs
Topics Featured
When developing critical assays, such as liquid biopsy-based cancer tests and pre-natal genetic screening kits, the composition of blood collection tubes used must be a major consideration.
Most blood collection tubes are made of either non-reactive glass or polyethylene terephthalate (PET). When targeting cell-free DNA (cfDNA), many have argued that plastic is superior to glass due to its clarity and durability. However, the effect that tube material has on preservative shelf life and blood draw volume is not often contemplated.
While we think that glass blood collection tubes are the best choice for cell-free DNA preservation, let’s walk through some data that supports our position.
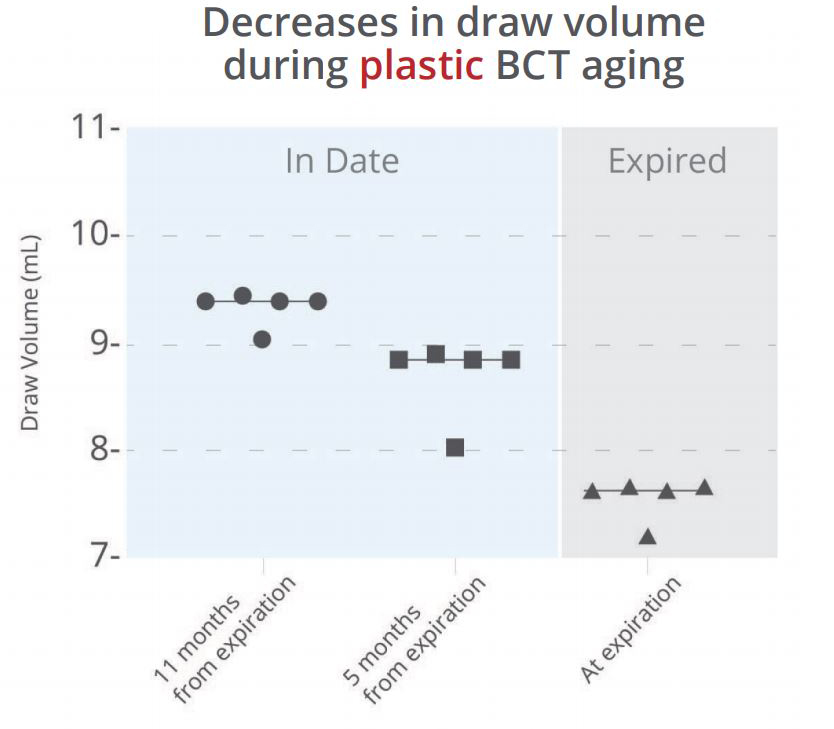
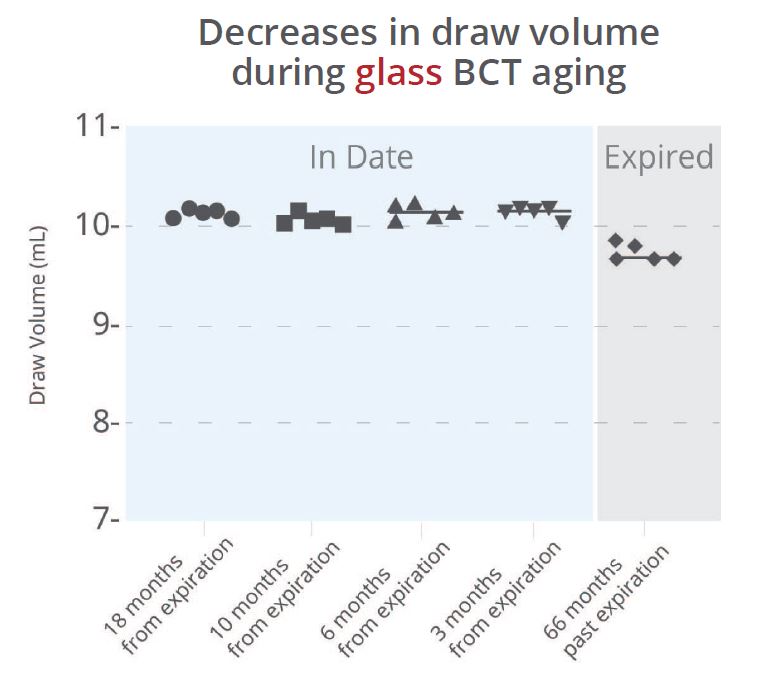
To examine whether the material of blood collection tubes affects blood draw volume, we collected water into five tubes for each of three lots of common plastic or glass blood collection tubes of various ages. A substantial change in the draw volume was observed from new to older plastic tubes, even when the older tubes were still “in-date” (Figure 1a). This decrease in draw volume can result in lower amounts of recoverable plasma, which may affect downstream assays or cause additional difficulty for rare target detection. In contrast, the amount of water drawn into the glass Cell-Free DNA BCT® remained constant throughout dating and for years after expiration (Figure 1b).
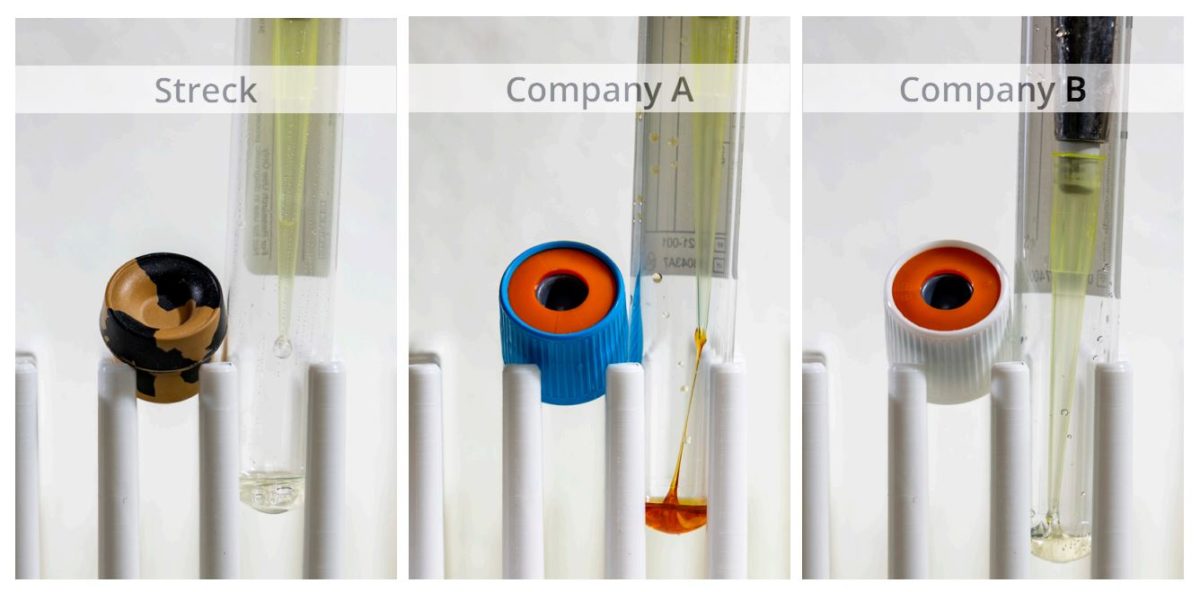
While most stabilizing reagents are a liquid at the time of manufacturing, they are prone to loss of moisture over time depending on which tube they are stored in. We observed increased reagent viscosity over time in plastic tubes compared to the glass Cell-Free DNA BCT, where reagent remained a liquid even years past expiration (Figure 2).
Overall, these data suggest that poor vacuum retention and loss of moisture not only shortens the shelf life of plastic blood collection tubes but can also lead to inconsistent results as the product ages. Glass blood collection tubes, such as Cell-Free DNA BCT, provide the best shelf life, performance near expiration and sample draw volume when stabilization is required.
Cell-Free DNA BCT is a direct-draw blood collection device intended for the collection, stabilization and transport of whole blood samples for use in conjunction with cell-free DNA next generation sequencing assays that have been cleared or approved for use with samples collected into the Cell-Free DNA BCT device.
Performance characteristics of this device have only been established on the Guardant360® CDx assay. RUO and CE-marked versions of Cell-Free DNA BCT are also available. Cell-Free DNA BCT RUO is For Research Use Only. Not for use in diagnostic procedures. Cell-Free DNA BCT CE is for Export Only. Not for use in the U.S.
How can nucleic acids predict disease?