CLIA Announces New 2024 Updates
Changes to CLIA requirements for Analytical Quality may affect your proficiency testing and quality control programs. Effective July 11, 2024, these r…
Read More With our sickle cell products, your laboratory can establish a quality control program for whole blood in vitro diagnostic testing methods. Our sickle cell line is composed of stabilized human red blood cells and can be handled in the same manner as a patient specimen.
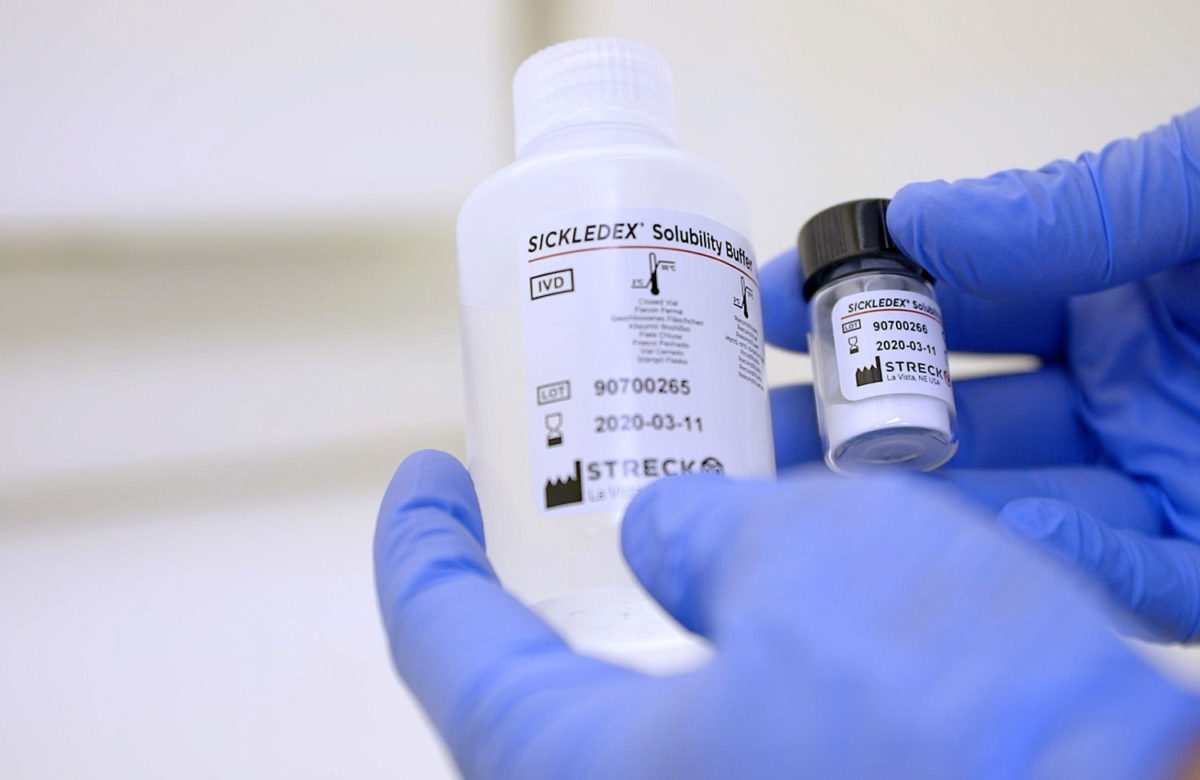
A positive and negative whole blood control available for use with sickle cell screening tests and hemoglobin electrophoresis…
A qualitative solubility test that can detect the presence of sickling hemoglobin in human blood samples within 6 minutes…