With the release of Lot #3359 on November 29, 2023, MDx-Chex for BCID2 will be configured into single-use vial format and a 12-month expiration date.
With the release of Lot #3359 on November 29, 2023, MDx-Chex for BCID2 will be configured into single-use vial format and a 12-month expiration date.
MDx-Chex® for BCID2
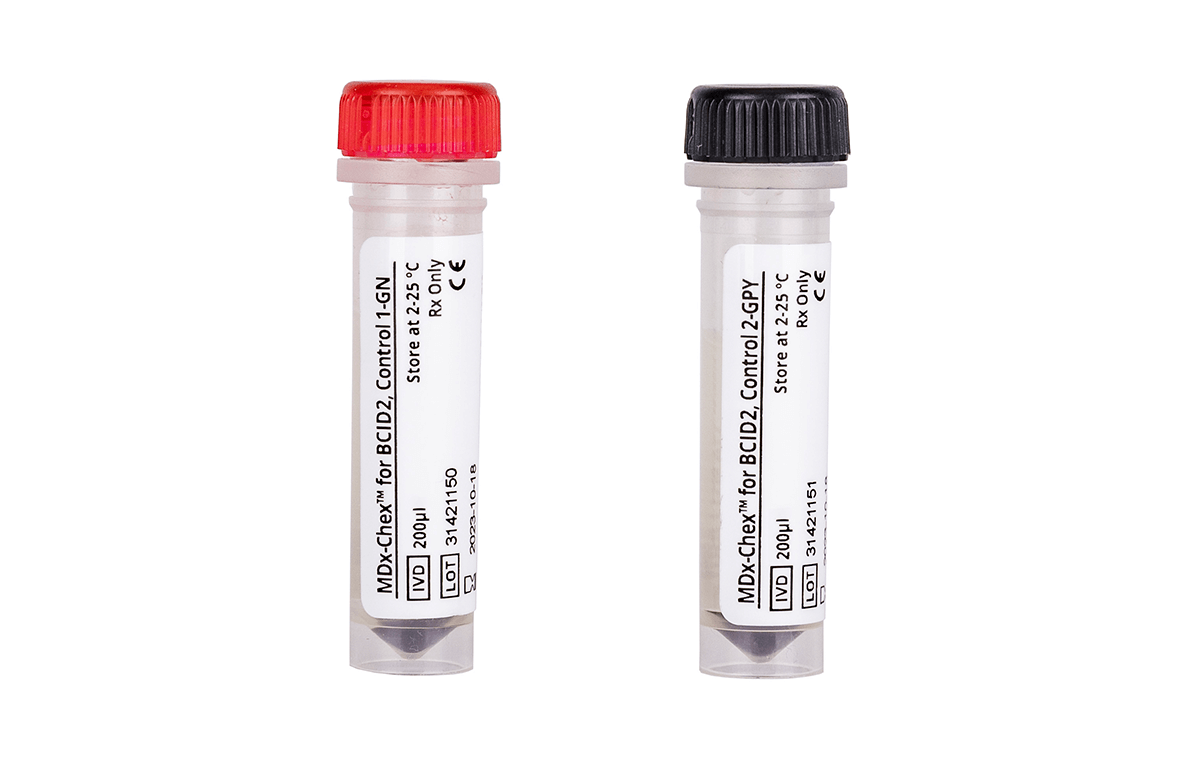
MDx-Chex for BCID2 is a first-of-its-kind quality control designed to evaluate the entire analytical process of the BIOFIRE® Blood Culture Identification 2 (BCID2) Panel for sepsis. With MDx-Chex for BCID2, you can track lot-to-lot performance of the BCID2 assay and reduce the occurrence of incorrect results due to instrument or assay failures.
Product Description
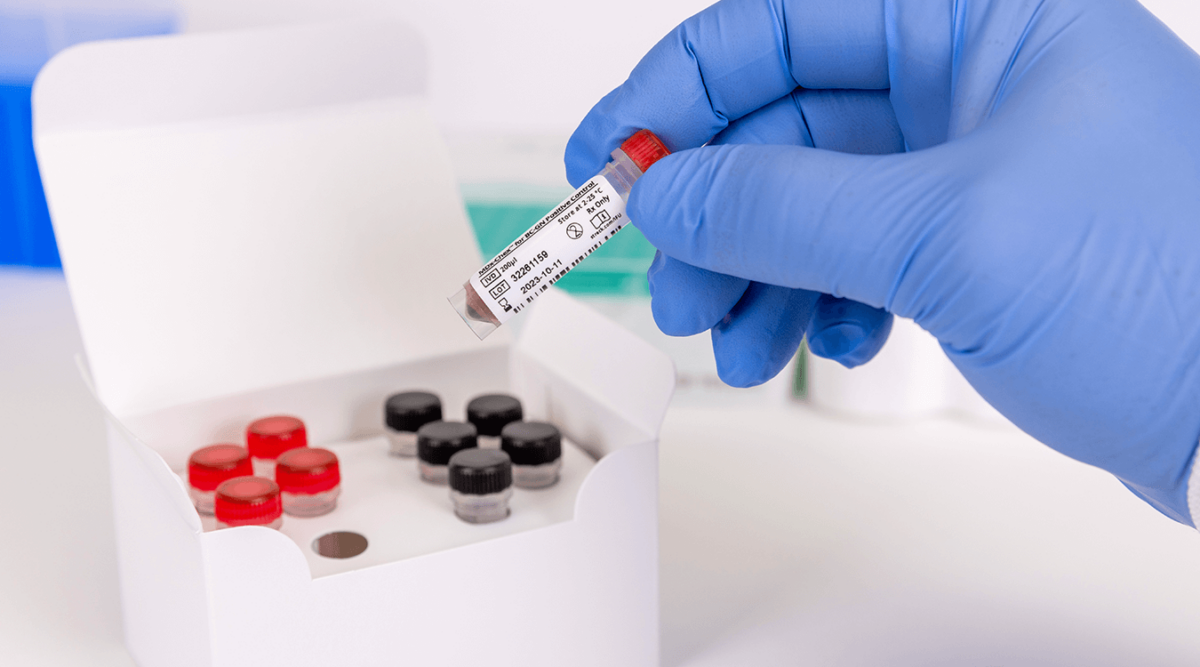
MDx-Chex for BCID2 is a patient-like full process control designed to validate the entire analytical process of the BIOFIRE BCID2 Panel, including cell lysis, DNA extraction, purification and removal of PCR inhibitors in addition to qPCR amplification, detection and analysis. MDx-Chex for BCID2 covers all targets tested on the BIOFIRE BCID2 Panel: 43 bacteria, yeasts and antimicrobial resistance gene targets packaged in 10 single-use vials, 5 for Gram (-) bacteria and 5 for Gram (+) bacteria and yeasts…
For In Vitro Diagnostics Use.
A step-by-step instruction for using MDx-Chex for BCID2 with the BIOFIRE FilmArray® 2.0 and Torch Systems.
ESCMID 2024 Presentation:
Molecular diagnostic tests: Why do you QC? Let’s get real…
Features
- Patient-like matrix of inactivated microorganisms, blood cells and culture media components
- Can be directly used on the BIOFIRE FilmArray® Torch and 2.0 platforms
- Provides complete coverage of all 43 BIOFIRE BCID2 Panel targets
Benefits
- Challenges and verifies the entire analytical process of the BIOFIRE BCID2 Panel
- Single-use vials reduce preanalytical variables such as pipetting errors and cross-contamination
- Easy to use – simply pipette like a patient sample
Benefits of MDx-Chex controls over other available controls | |||
Streck | Competitor A | Competitor B | |
Preanalytical Variables | |||
Sample tracking (via barcode)^ | Yes | No | No |
Controls technician sample handling | Yes | No | No+ |
Preanalytical Variables | |||
Lysing | |||
Contains non-infectious intact organisms | Yes | No | Yes |
Contains human RBCs and WBCs | Yes | No | No |
Lyses all panel organisms | Yes | No | Yes* |
Nucleic Acid Isolation and Purification | Yes | No | No |
Patient-like sample matrix | Yes | No | No |
Inhibitors | |||
RBCs (i.e., hemoglobin) | Yes | No | No |
WBCs (i.e., interleukin, human genomic DNA) | Yes | No | No |
Culture media chemicals | Yes | No | No |
Specificity | |||
All targets present | Yes | Yes** | Yes* |
Off target (human gDNA) present | Yes | No | No |
Detection | |||
All DNA targets | Yes• | Yes | Yes* |
FDA Class II Clearance | Yes | No | No |
+ Requires pooling of multiple components, not part of patient sample testing workflow
* If all panel organisms are pooled
• Streck controls contain all DNA targets for the BIOFIRE BCID2 Panel
** When Lyophilized pellets are rehydrated and pooled together
^ Barcode allows the control lot number, expiration date and level to be captured in the software
Ordering Information
| Description | Catalog Number |
|---|---|
| 5 Gram (–) bacteria and 5 Gram (+) bacteria vials, 5 run capacity | 250065 |
Order MDx-Chex for BCID2 today
New customers
Existing customers
International customers find a distributor
Resources
Instructions (IFU)
Product Information
Resources
Looking for an older document? Click here for archived assays and certificates.
Molecular Products
With full-process controls and antibiotic resistance kits, Streck has the products to help you streamline your molecular diagnostics testing, aid antibiotic stewardship and ensure positive patient outcomes.
The latest from the blog
New antibiotic fights antimicrobial resistant bacteria