Flow Cytometry: A powerful tool for clinicians
Topics Featured
Flow cytometry allows scientists to rapidly analyze thousands of normal cells and identify the presence of rare and abnormal cells. It is based on simple principles that, when combined, form a powerful analytical tool.
The invention of flow cytometry
For early scientists, the only laboratory tool available to “see” red and white blood cells was optical microscopy. However, in 1953, the properties of laminar flow were harnessed to develop a new method for the assessment and characterization of biological tissue at the cellular level. While the first flow cytometers could only measure cell size by impedance calculations based on the Coulter principle, in the 1970s, fluorescently labeled antibodies and sensitive detectors were introduced to the process, and modern flow cytometry was born.
In the decades since its invention, flow cytometry has flourished. Now, flow cytometry is a powerful diagnostic procedure that can identify and quantify up to 30 or more cell parameters at a time.
What is flow cytometry?
Flow cytometry depends on the use of fluorescently labeled antibodies that bind to antigens of interest on the cell. Cells are stained with antibodies then suspended in a neutral fluid and analyzed on the flow cytometer. Once within the flow cytometer, cells are organized by laminar flow and pass through a chamber, called a flow cell, one cell at a time. As the cells travel through the flow cell, they pass a laser beam which excites the conjugated fluorophore. Each time a cell passes through the laser, the light is refracted based on the cell’s size, and the flow cytometer records an event. The light-scatter from the cell and emitted fluorescence from the antibody-fluorophore combination are collected by optical detectors and transferred to a digital format to be analyzed on a dot plot or histogram.
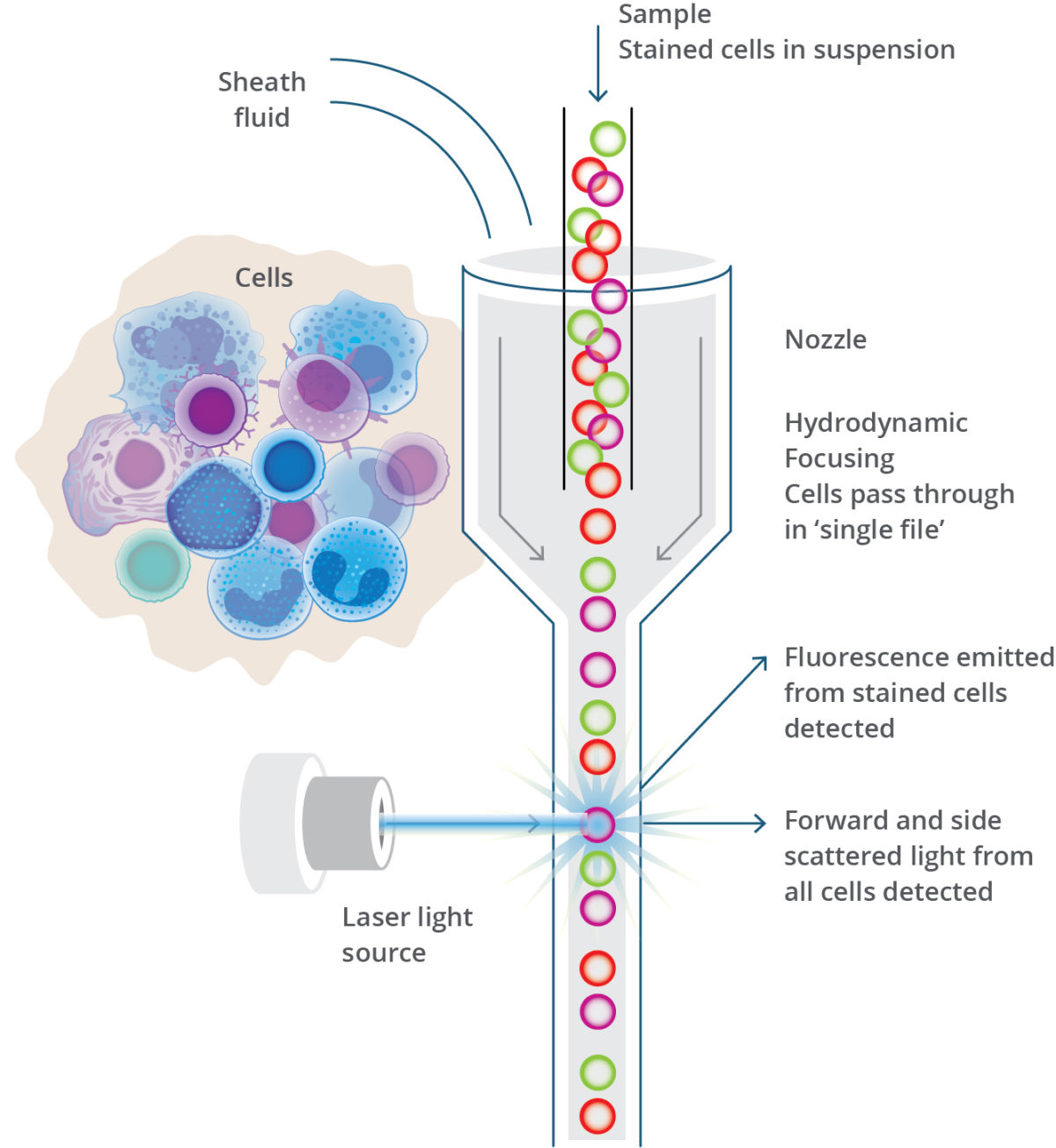
Light scatter collected by forward (FCS) and side scatter (SSC) creates patterns that are characteristic of the size and internal complexity of the cell. This indicates what type of cells (ex., lymphocyte, monocyte, granulocyte, platelet) are present. Fluorescent-labeled antibodies identify the cell’s surface and cytoplasmic antigens, often called clusters of differentiation (CD) markers. Aberrant expression of the CD markers is cause for investigation by clinicians, as these abnormalities can suggest serious health issues.
Flow cytometry can detect, identify and measure multiple characteristics of an individual cell from virtually any kind of biological sample, including:
- Peripheral blood
- Cerebrospinal fluid (CSF)
- Bone marrow
- Biopsies from tumors and lymph nodes
- Urine
Flow Cytometry Vocabulary
Antibody ‒ a protein produced by the immune system in response to an antigen. The antibody recognizes and binds to this substance and aids the immune system in targeting the foreign substance for destruction.
Antibody Titration ‒ a method of determining the correct amount of fluorophore conjugated antibodies to use in order to get the best resolution for each antigen and cell of interest.
Antigen ‒ generally a foreign substance, such as a chemical structure, bacterial protein, virus or pollen, that can stimulate a specific immune response.
Autofluorescence ‒ non-specific fluorescence emission observed when certain cellular molecules are excited by a particular wavelength and emit light that is detected by the flow cytometer.
Clones ‒ genetically identical daughter cells from one unique parent cell.
Clusters of Differentiation (CD) ‒ a nomenclature system given to proteins characteristic to immune cells (mostly surface proteins) in order to standardize naming. CD molecules may be specific for a single type of cell or cell lineage or may be found on several immune cells. The official listing of determinants has identified more than 400 individual and unique markers and continues to grow.
Density Plot ‒ graphical representation of two-parameter data where the colors represent the number of events at that location. Each axis represents the single intensity of one parameter.
Dot Plot ‒ graphical representation of two-parameter data where each axis represents the single intensity of one parameter and each dot represents a single cell’s level of that parameter.
Event ‒ an individual particle, generally a cell, that goes through the laminar flow cell and is interrogated by the flow cytometer for size, granularity and fluorescence levels.
Fluorophore/Fluorochrome ‒ a molecule that accepts light at a certain wavelength (excitation) and re-emits it at a longer wavelength (emission), producing fluorescence.
Gates ‒ numerical or graphical boundaries that are drawn around cells that share characteristics such as forward light scatter (FCS), side light scatter (SSC) and marker (CD) expression for further analysis.
Histogram ‒ a graphical representation of single-parameter data that shows relative number and distribution of events (or cells). The horizontal axis corresponds to the single intensity of a selected parameter, while vertical axis represents the number of events (counts).
Immunophenotyping ‒ the detection of specific cell types by using flow cytometry to identify immune-related markers on the cell surface. This is the basis of the most common clinical uses of flow cytometry.
Laminar Flow ‒ movement of liquid where each layer of fluid moves past adjacent layers with very little to no mixing.
Resolution ‒ the potential of a particular flow cytometry instrument to detect differences in fluorescence frequencies.
Quality Control for Clinical Flow Cytometry
Clinical labs must maintain CAP accreditation and CLIA compliance by running positive procedural controls to verify the performance of reagents, preparation methods, staining procedures and instrument performance. While these positive controls can be derived from patient samples, even qualitative leukemia and lymphoma control testing requires a lab to establish an acceptable target and range for unassayed control material through repetitive analysis that includes previously tested control material (i.e., a lot-to-lot conversion). Readily available, third-party control material provides consistent values, eliminating the need for the user to establish and validate assay ranges and saving laboratories valuable time and money in the long-term.
We offer a comprehensive line of positive procedural controls and cellular stabilization products for immunophenotyping by flow cytometry. Our CD-Chex® quality controls provide you with assay values for various leukocyte populations and reference values for intracellular and surface markers, and our cellular stabilization products allow you to preserve samples for extended periods of time, eliminating the need for cold-chain shipping or immediate processing.


ILQC: How to earn the trust of your clients and their patients