microRNA in RNA Complete BCT
Topics Featured
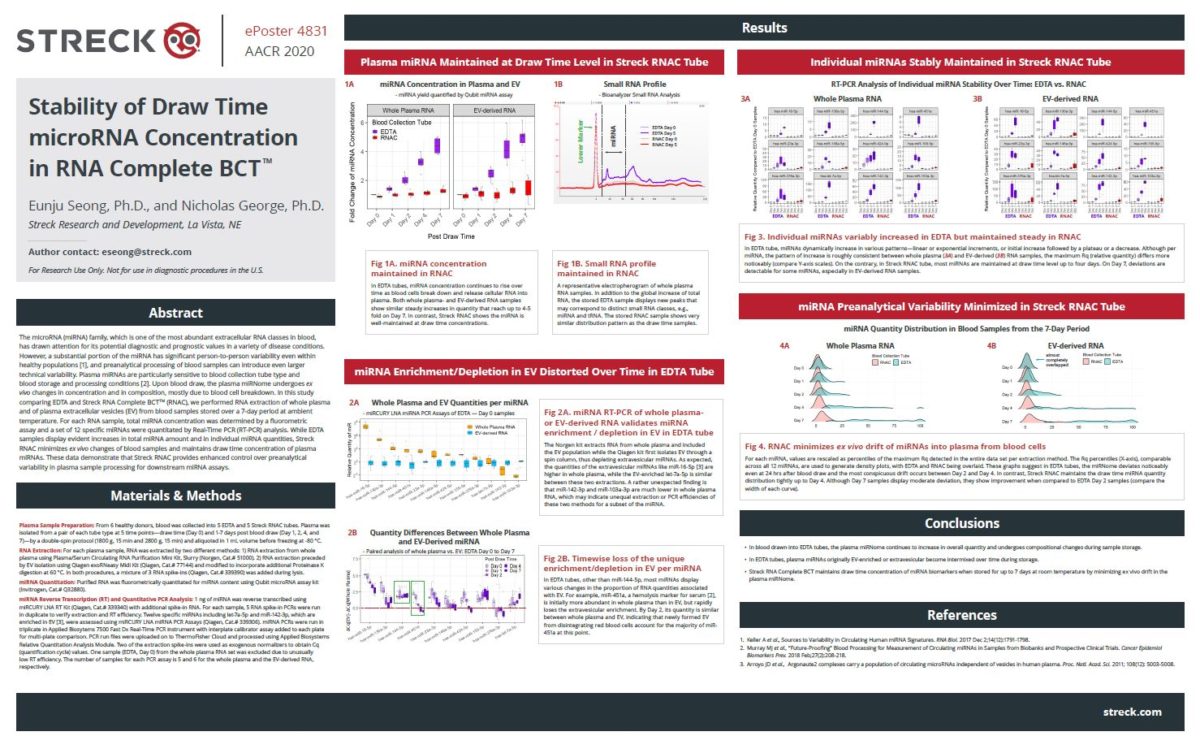
Streck recently presented data on our new and innovative blood collection tube, RNA Complete BCT®, at the American Association for Cancer Research (AACR) Annual Meeting II. In ePoster #4831 titled “Stability of draw time microRNA concentration in RNA Complete BCT,” Streck R&D scientist Dr. Eunju Seong discusses plasma microRNA, which has drawn attention for its biomarker potential in various disease conditions. This study determined that the Streck tube minimizes changes in microRNA concentration (or changes to the microRNAome), thus facilitating detection of disease-specific microRNAs after ambient storage for up to 7 days.
Poster overview
MicroRNA (miRNA) is small non-coding RNA, usually only 21-23 nucleotides long, which regulates gene expression through targeting translational repression or mRNA degradation. miRNA is one of the most abundant and stable extracellular RNAs in body fluids. In blood, some microRNAs are encapsulated inside extracellular vesicles (EVs) and others are bound to protein carriers, independent of EVs, that freely circulate. Aberrant expression of miRNAs is associated with a variety of human diseases such as cancer, diabetes, viral infections and neurodegenerative disorders. The expression of miRNAs can reflect pathological changes in many diseases and their expression profiles may provide novel diagnostic and prognostic values. Particularly in cancer, miRNA can be used to identify both cancer type and tissue origin.
However, a substantial portion of miRNA displays significant person-to-person variability even within healthy populations and age seems to be an additional confounding factor. Pre-analytical processing of blood samples can introduce even larger technical variability in miRNA, which is an additional hurdle to overcome in diagnostic assay development. Plasma miRNAs are known to be especially sensitive to blood sample collection and storage, plasma isolation, and RNA extraction methods. Pursuant to this, we found blood samples collected in conventional EDTA tubes have plasma miRNAome tainted with by-products of blood cell disintegration which is initiated upon blood collection. Further, total plasma miRNA concentration continued to rise during storage. We selected a set of 12 specific miRNAs, two of which primarily originated from the EV associated population and analyzed their quantity in plasma-derived or EV enriched fractions by Real-Time PCR analysis. All 12 miRNAs showed increases over time, in various patterns.
Recently, Streck has developed a new blood collection tube, RNA Complete BCT, which maintains the cell-free mRNA population. Based on changes observed in EDTA, we examined if RNA Complete BCT could also maintain plasma miRNA. In contrast to the plasma samples from EDTA tubes, RNA Complete BCT derived plasma or EV samples maintained the draw time total miRNA concentration throughout a 7-day room temperature storage period. Real-Time PCR analysis of the same 12 miRNAs previously mentioned confirmed the individual miRNAs are maintained at the draw time level up to seven days.
These results demonstrate that Streck RNA Complete BCT minimizes ex vivo changes in blood samples and maintains the draw time concentration of plasma miRNA biomarkers. Streck RNA Complete BCT will provide enhanced control over pre-analytical variability in plasma sample processing and can help with the development of microRNA assays.

Dr. Seong received her Ph.D. in Neuroscience from the University of Michigan Ann Arbor and joined Streck in 2019. Applying her prior research experiences in Human Genetics and Cell Biology to liquid biopsy, she studies pre-analytical variables associated with disease-implicated bioanalytes including exosomes and circulating microRNA.
RNA Complete BCT is Research Use Only. Not for use in diagnostic procedures in the U.S. A CE version of these tubes is also available. RNA Complete BCT CE is for Export Only. Not for sale in the U.S.

FDA clearance brings liquid biopsy into a new era