Key development for liquid biopsy research
Topics Featured
New publication seeks to normalize liquid biopsy sample handling to advance cancer research
A recent publication from the BloodPAC group seeks to standardize pre-analytical variables for liquid biopsies. Titled “Minimum Technical Data Elements for Liquid Biopsy Data Submitted to Public Databases,” this peer-reviewed and CAP-approved structure details the best practices for data collection. The working group details the 11 most important pre-analytical attributes when it comes to liquid biopsy research and sample handling. Through the unification of pre-analytical variable handling, data can be considered from a level playing field, which is critical for advancement.

Minimum Technical Data Elements for Liquid Biopsy Data Submitted to Public Databases
Background
Pre-analytical variables are an undoubtedly important aspect of any well-designed study, but all too often researchers will deal with issues such as sample collection, handling, shipping and processing time frames after an error has occurred. This is not hard to understand, as most studies start small, with low numbers of inputs and outputs. However, as studies grow beyond the initial scope, gain wider footprint, and increase in complexity, the project can hit the pre-analytical variable wall. For example, it is often trivial to find the time to process one crucial donor sample in a timely fashion versus tens or hundreds of precious samples. Along the same lines, one person performing all the work is dramatically different when scope expands to a team doing the work in tandem.
New fields often struggle with identification of the best practice to track these kinds of variations, and critical data can easily be left behind when important variables are left out of manuscripts and databases. As an answer to this problem specifically in liquid biopsy, a collaborative community has evolved in the form of BloodPAC.
What is BloodPAC?

The Blood Profiling Atlas in Cancer (BloodPAC) Consortium is an organization of academic, commercial, nonprofit, public and regulatory groups united behind the mission of advancing the field of liquid biopsy to improve the outcomes of patients with cancer.
The BloodPAC mission statement: “Our mandate is to accelerate the development, validation, and clinical use of liquid biopsy assays to better inform medical decisions so that patient outcomes are improved. To do so, we have developed a collaborative infrastructure that enables sharing of information between stakeholders in the public, industry, academia, and regulatory agencies.”
What are the recommended pre-analytic data elements?
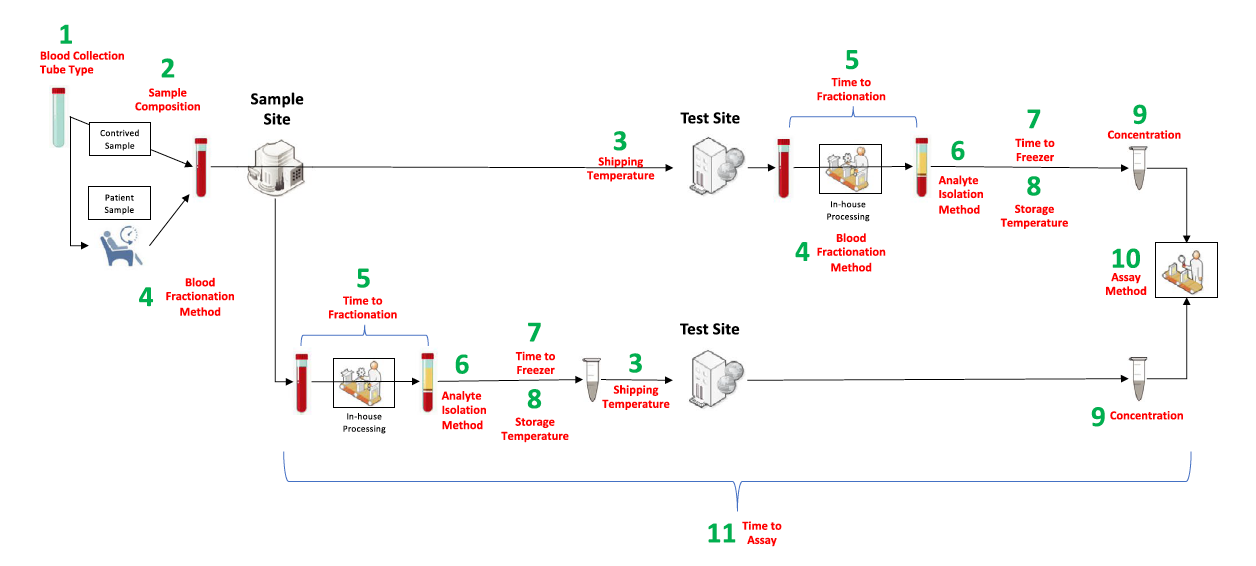
BloodPAC developed recommendations for 11 pre-analytical attributes called the Minimum Technical Data Elements (MTDEs) that are essential for studies that it sponsors and for data contributed to the BloodPAC Data Commons.
Click here to view the paper that describes the steps taken by BloodPAC to develop this comprehensive list of pre-analytical variables relevant to cfDNA-based tests.
1. Blood collection tube type
2. Sample composition
3. Shipping temperature
4. Blood fractionalization method
5. Time to fractionation
6. Analyte isolation method
7. Time to freezer
8. Storage temperature
9. Concentration: cellular concentration or molecular concentration
10. Assay method
11. Time to assay
Streck continues to be a proud member of the BloodPAC consortium and recognizes the value of limiting pre-analytical variables in critical studies to advance fields of research such as liquid biopsy. In supporting our customers and assuring confidence in their samples, Streck stabilization products allow scientists to focus on the assay and their next research discovery, instead of questioning if their samples are trustworthy.

FDA clearance brings liquid biopsy into a new era