FDA drafts guidance on ctDNA
Topics Featured
Earlier this year, the U.S. Food and Drug Administration released a draft guidance regarding the use of circulating tumor DNA (ctDNA) for early-stage solid tumor drug development. ctDNA is a kind of cell-free DNA (cfDNA) derived from cancer cells and tumor cells circulating in the bloodstream.
ctDNA has been extensively studied for its use as a non-invasive biomarker in both detecting metastatic cancer and monitoring cancer treatment. As shown in the figure below, ctDNA can be used for early detection of cancer1, monitoring treatment response2, detecting minimal residual disease3, monitoring cancer recurrance4, and assessing for therapy resistance mutations5. Although ctDNA has great potential, few studies explore the clinical and regulational applications of utilizing ctDNA in early-stage, non-metastatic solid tumors.
Potential use of ctDNA as biomarker in oncology
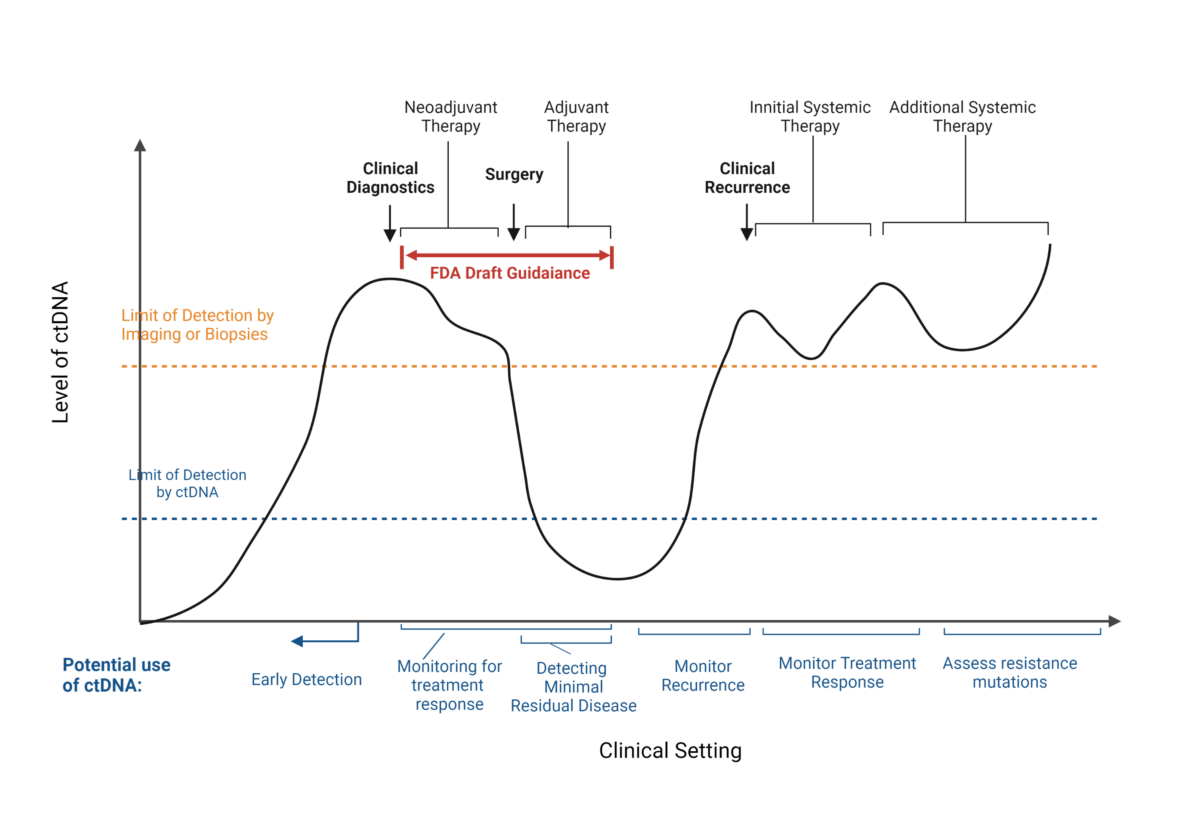
The new FDA guidance focuses on the potential uses of ctDNA in clinical trials for (neo)adjuvant therapy (see figure above). The FDA recognizes that drug trials for early-stage, non-metastatic solid tumors typically involve large sample sizes, long duration and high costs. In the draft, the FDA provides applicable uses of ctDNA to assist and expedite these drug trials.
For instance, drug companies may enrich clinical samples obtained from high- and low-risk patients based on molecular alteration (i.e., genetic or epigenetic changes in ctDNA) or risk of residual disease (levels of ctDNA). A homogenous patient population is anticipated to help reduce trial size and length. Furthermore, ctDNA could be used to help determine drug activity and efficacy during early phase clinical trials and may even be used as an early endpoint in trials, similar to measures like disease-free survival, event-free survival, overall survival and pathologic complete response.
Wendy Royalty, Streck’s Director of Quality Assurance & Regulatory Affairs, welcomes the draft guidance. “The FDA draft industry guidance on the use of ctDNA for early-stage solid tumor drug development is very exciting. It shows the FDA’s recognition of the potential value of using ctDNA to bring essential early-stage cancer treatment drugs to market more expeditiously compared to the current very lengthy timelines.”
FDA’s draft guidance also suggested that further data collection is required to grant approval for these types of use and encourages the industry to be proactive in developing the appropriate type of data generation. Specifically, the FDA recommends that trial sponsors consider a standardized protocol for “sample collection, storage, processing and handling.”
This is critical as plasma ctDNA concentration may vary due to differences in pre-analytical processing, including the tube type used for whole blood collection as well as the associated storage conditions. Without proper stabilization, both the population and concentration of ctDNA will change significantly6, making it difficult or impossible to effectively detect ctDNA changes reflective of long-term treatment outcomes.

When developing their ctDNA assays, drug sponsors may consider using Streck’s Cell-Free DNA BCT® RUO as one way to reduce pre-analytic variation. Cell-Free DNA BCT RUO, long used by academic researchers and assay developers for ctDNA research, is a direct-draw venous blood collection device designed to maintain the draw-time cfDNA concentration and population during the collection and transport of samples. Cell-Free DNA BCT is For Research Use Only, not for use in diagnostic procedures.
References:
- Herrera AF, Tracy S, Croft B, Opat S, Ray J, Lovejoy AF, Musick L, Paulson JN, Sehn LH, Jiang Y. Risk profiling of patients with relapsed/refractory diffuse large B-cell lymphoma by measuring circulating tumor DNA. Blood Adv. 2022 Mar 22;6(6):1651-1660.
- Gao Z, Shang Y, Wang X, Ma Y, Yang F, Wang J, Chen K, Zhang Y. Application of circulating tumor DNA for dynamic monitoring of advanced non-small cell lung cancer treatment response: An open-label, multicenter, prospective, observational study protocol. Thorac Cancer. 2019 May;10(5):1310-1315.
- Dasari, A., Morris, V.K., Allegra, C.J. et al. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal–Anal Task Forces whitepaper. Nat Rev Clin Oncol 17, 757–770 (2020).
- Qiao G, Zhuang W, Dong B, Li C, Xu J, Wang G, Xie L, Zhou Z, Tian D, Chen G, Tang J, Zhou H, Zhang D, Shi R, Chen R, Nian W, Zhang Y, Zhao J, Wen X, Xu Y, Li B, Zhang Z, Cai S, Ben X, Qi Y. Discovery and validation of methylation signatures in circulating cell-free DNA for early detection of esophageal cancer: a case-control study.
- Berger F, Marce M, Delaloge S, Hardy-Bessard AC, Bachelot T, Bièche I, Pradines A, De La Motte Rouge T, Canon JL, André F, Arnould L, Clatot F, Lemonnier J, Marques S, Bidard FC; PADA-1 investigators. Randomised, open-label, multicentric phase III trial to evaluate the safety and efficacy of palbociclib in combination with endocrine therapy, guided by ESR1 mutation monitoring in oestrogen receptor-positive, HER2-negative metastatic breast cancer patients: study design of PADA-1. BMJ Open. 2022 Mar 3;12(3):e055821.
- Risberg B, Tsui DWY, Biggs H, Ruiz-Valdepenas Martin de Almagro A, Dawson SJ, Hodgkin C, Jones L, Parkinson C, Piskorz A, Marass F, Chandrananda D, Moore E, Morris J, Plagnol V, Rosenfeld N, Caldas C, Brenton JD, Gale D. Effects of Collection and Processing Procedures on Plasma Circulating Cell-Free DNA from Cancer Patients. J Mol Diagn. 2018 Nov;20(6):883-892.

The Benefits of Glass BCTs