Circulating RNA and EV poster
Topics Featured
Streck recently presented data on our new and innovative blood collection tube, RNA Complete BCT™, at the American Association for Cancer Research (AACR) Annual Meeting II. In ePoster #759 titled “RNA Complete BCT: A novel blood collection tube targeting circulating RNA and extracellular vesicles,” Dr. Nick George explains that donor specimen integrity is a significant hurdle in making research-based liquid biopsy testing with EVs and cfRNA commonplace. Results from the study showed Streck RNA Complete BCT will allow for delayed sample processing without effect on sample integrity or downstream analyte analysis.
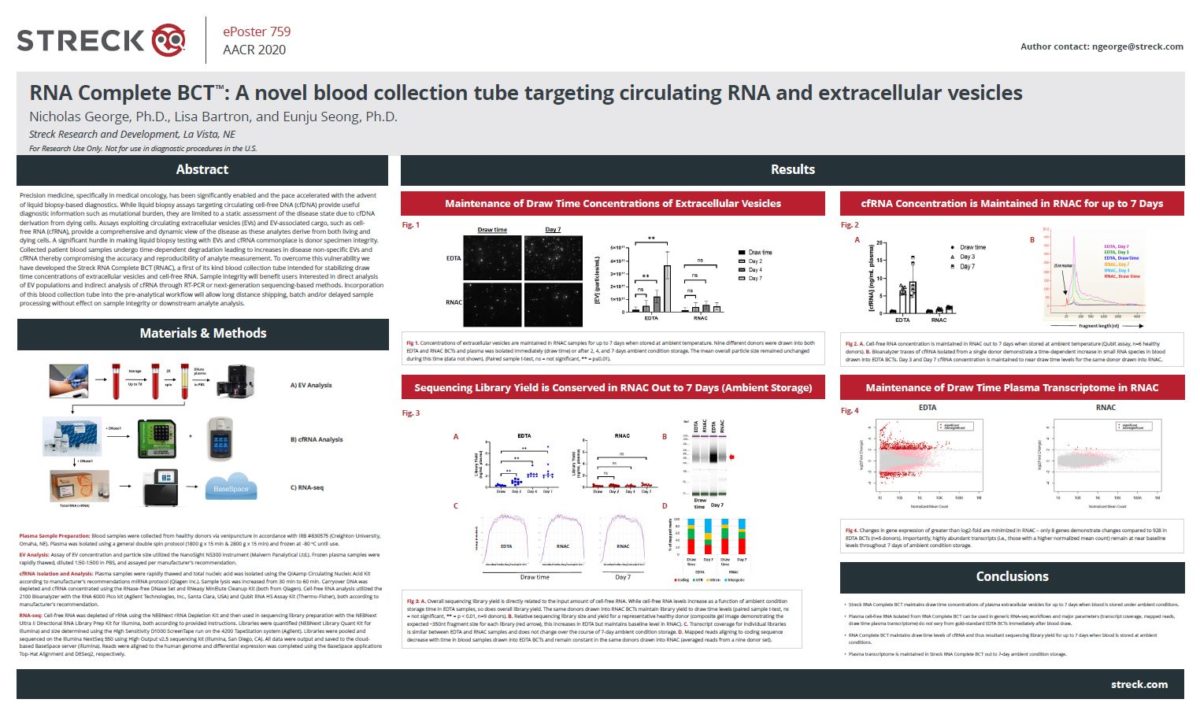
RNA Complete BCT: A novel blood collection tube targeting circulating RNA and extracellular vesicles
View the poster to learn how incorporation of RNA Complete BCT into the pre-analytical workflow will allow for delayed sample processing without effect on sample integrity or downstream research analyte analysis.
Poster overview
Precision medicine has been significantly enabled and pace accelerated with the advent of liquid biopsy. While liquid biopsy assays targeting cell-free DNA provide useful diagnostic information, such as cancer mutational burden, they are limited to a static assessment of the disease state. Research-based assays that exploit extracellular vesicles (EVs) and EV-associated cargo, such as cell-free RNA (cfRNA), provide a comprehensive and dynamic view of the disease as these analytes derive from both living cells and dying cells. A significant hurdle in making research-based liquid biopsy testing with EVs and cfRNA commonplace is donor specimen integrity. Collected patient blood samples undergo time-dependent degradation leading to increases in disease non-specific EVs and cfRNA thereby compromising the accuracy and reproducibility of analyte measurement. To overcome this vulnerability we have developed the Streck RNA Complete BCT. The Streck RNA Complete BCT is a research use only product.
Our AACR 2020 poster describes the developmental strategy for the RNA Complete BCT. The central hypothesis stemmed from early studies demonstrating that the majority of cfRNA in plasma, specifically messenger RNA, is found within EVs, for example exosomes and microvesicles, thus protecting it from degradation. Our results demonstrate that the RNA Complete BCT maintains draw time concentrations of EVs in whole blood samples out to 7 days of ambient storage. Stable EV concentration in turn results in limited change in cfRNA concentration for the sample storage period. We then tested the functionality of the RNA Complete BCT using an in-house developed plasma RNA-seq workflow. Similar assays are in research development by numerous groups for disease detection and monitoring. A critical factor here is the sequencing library and its preparation from blood plasma. Any increases or decreases in yield are the direct result of changes in the blood sample (e.g., hemolysis or cellular breakdown) or cfRNA isolation. Importantly, the amount of input cfRNA dictates overall library yield. To maintain the sensitivity of a given assay, one should maintain draw time levels of cfRNA and thus library yield – any increases dilute the sequencing reads specific to a given disease analyte. Our results demonstrate efficient maintenance of draw time cfRNA levels and resultant sequencing library yield. Finally, direct comparison of gene expression changes demonstrate that the RNA Complete BCT robustly maintains the draw time plasma gene expression profile out to 7 days, while the EDTA control does not. This is critical for RNA-seq-based protocols aimed at identifying subtle changes in disease-specific gene expression.
Altogether, our results highlight a valuable solution to the pre-analytical workflow for EV and cfRNA research – the Streck RNA Complete BCT. Intended uses include direct analysis of EV populations and indirect analysis of cfRNA through RT-PCR or next-generation sequencing-based methods. Incorporation of this blood collection tube into the pre-analytical workflow will allow for delayed sample processing without effect on sample integrity or downstream research analyte analysis.

Dr. George received his Ph.D. in Cancer Biology from the University of Nebraska Medical Center and joined Streck in 2015. His current research and development interests include facilitating research into liquid biopsy and the multitude of pre-analytical variables associated with sample collection, processing and analysis.
RNA Complete BCT is Research Use Only. Not for use in diagnostic procedures in the U.S. A CE version of these tubes is also available. RNA Complete BCT CE is for Export Only. Not for sale in the U.S.

FDA clearance brings liquid biopsy into a new era