Abstract Control Number: 673 | AACR 2025
Streck Research and Development, La Vista, NE
Protein Plus BCT™: Ensuring reliable protein biomarker analysis in liquid biopsies by minimizing pre-analytical variations
Rachel M. Miller, Ph.D., Sama Mehta, Jing Li, Ph.D.
INTRODUCTION
While plasma protein biomarkers show promise for cancer early detection, diagnosis, prognosis, and monitoring, pre-analytical variation can affect sample integrity and limit the reliability of proteomic analysis. Sample collection, storage, and processing conditions can alter the proteome through ex vivo blood cell activation and lysis, which releases proteins that can obscure relevant in vivo abundances. This can significantly impact cancer biomarkers, many of which are expressed in white blood cells and platelets. Here, we use proteomics to investigate the impact of plasma processing and storage conditions on the plasma proteome, and the impact the use of Protein Plus BCT has on proteome alterations.
Protein Plus BCT is for Research Use Only. Not for use in diagnostic procedures. Protein Plus BCT should only be used for research or the development of new assays.
METHODS
Blood was collected from three healthy donors into EDTA and Protein Plus BCT. Plasma was isolated using single- (1300 xg for 10 min) and double-spin (1800 xg for 15 min, 2800 xg for 15 min) protocols immediately after draw, after 3 days of storage at either 2 °C, room temperature (22 °C), or 37 °C, or after 5 days of ambient temperature storage (22 °C). Samples were prepared for bottom-up proteomic analysis using the Seer Proteograph™ XT workflow and analyzed using data-dependent acquisition on an Orbitrap™ Exploris™ 240 mass spectrometer (Thermo Scientific™). Data was analyzed using MetaMorpheus and Perseus. Samples were also prepared for immunoassay analysis with the Ella™ Simple Plex™ Assay for TGF-β1 (Bio-Techne®).
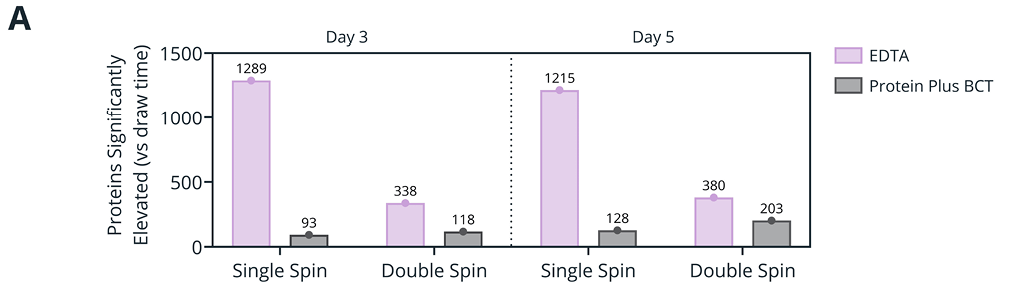
Impact of plasma isolation spin protocol on the plasma proteome
Double-spin plasma isolation protocol minimizes proteome variation relative to single-spin protocol
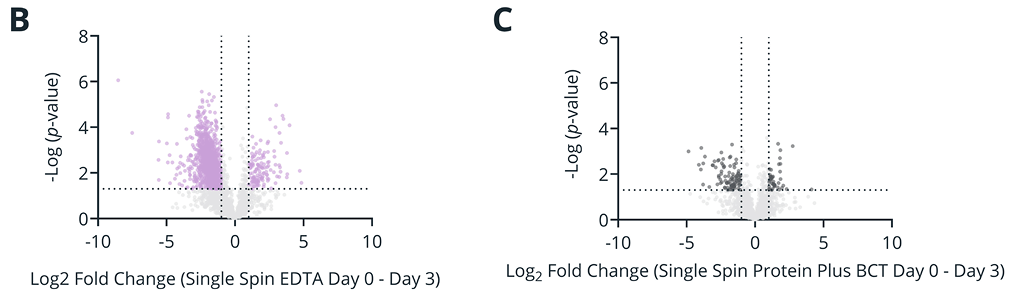
Figure 1. (A) Number of proteins significantly elevated in abundance after 3 or 5 days of whole blood storage relative to draw time (day 0) when plasma was isolated with single- or double-spin protocol. (B-E) Differential analysis of plasma protein abundances between plasma isolated from EDTA (B, D) or Protein Plus BCT (C, E) blood after 3 days of whole blood storage compared to draw time for plasma isolated by single- (B-C) or double-spin (D-E) protocols. Colored markers: Log2 fold change >1 or < -1, -Log(p-value) > 1.3. All quantitative analysis was performed for proteins quantified in at least 70% of the samples for each condition (single spin: 2983, double spin: 2504 proteins).
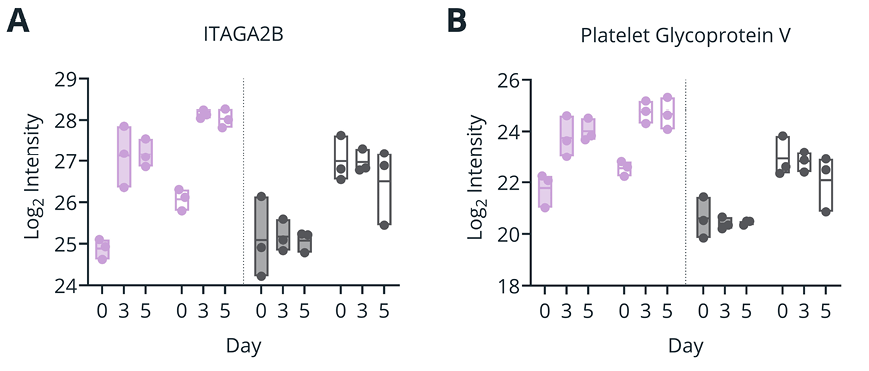
Impact of spin protocol on blood cell-associated proteins
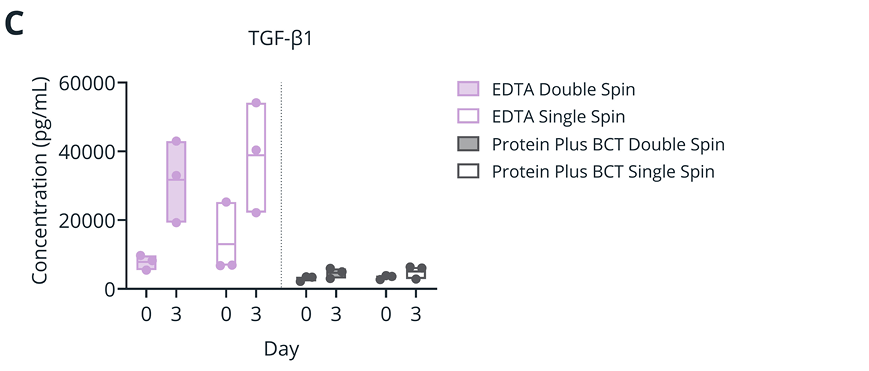
Figure 2. (A-B) Plasma levels (Log2 Intensity, by mass spectrometry) of platelet-associated proteins ITGA2B (A) and Platelet Glycoprotein V (B) for samples isolated with double- and single-spin protocols at draw time, or after 3 and 5 days of ambient temperature storage. (C) Plasma TGF-β1 concentration (pg/mL) as determined by immunoassay for samples isolated with double- and single-spin protocols at draw time or after 3 days of ambient temperature storage.
Protein identifications differ between plasma isolated by single- and double-spin protocols
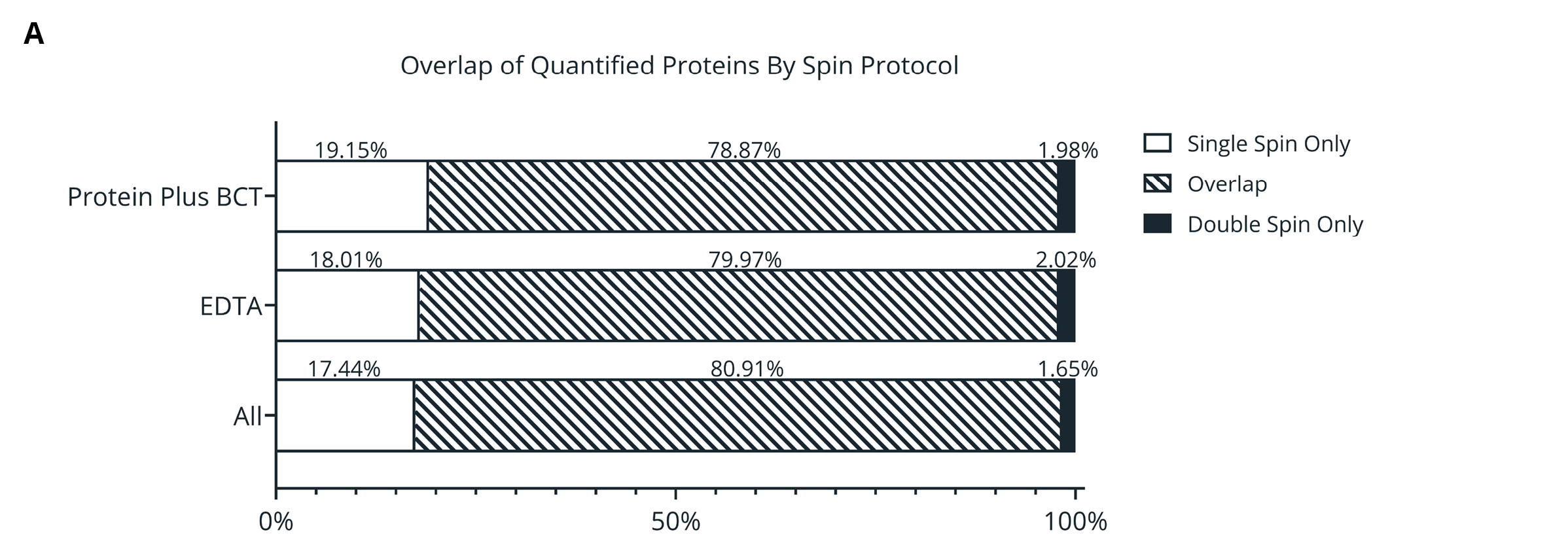
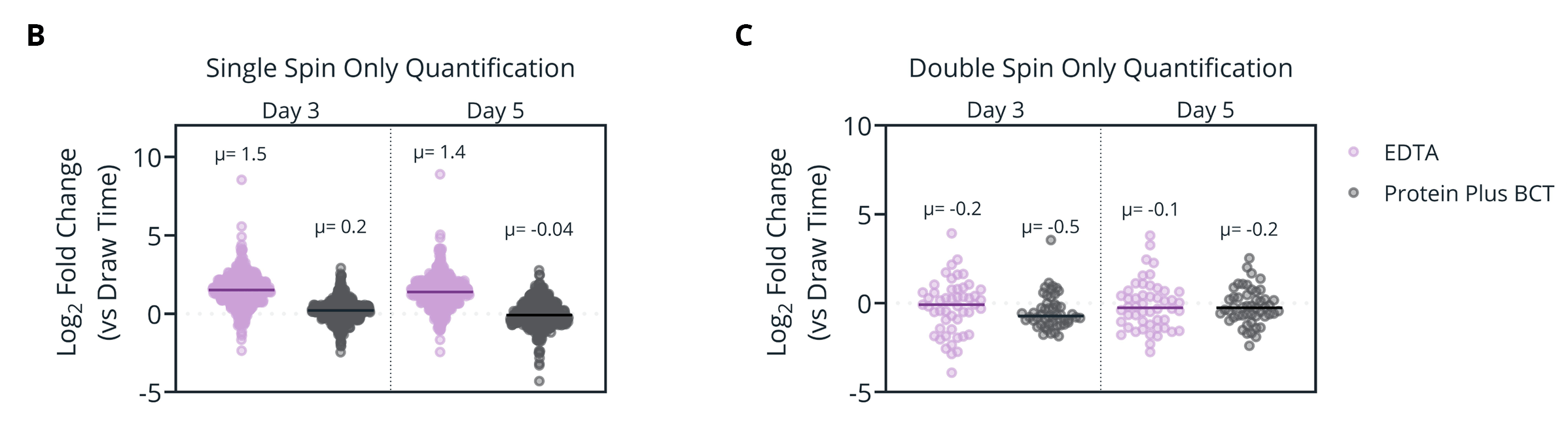
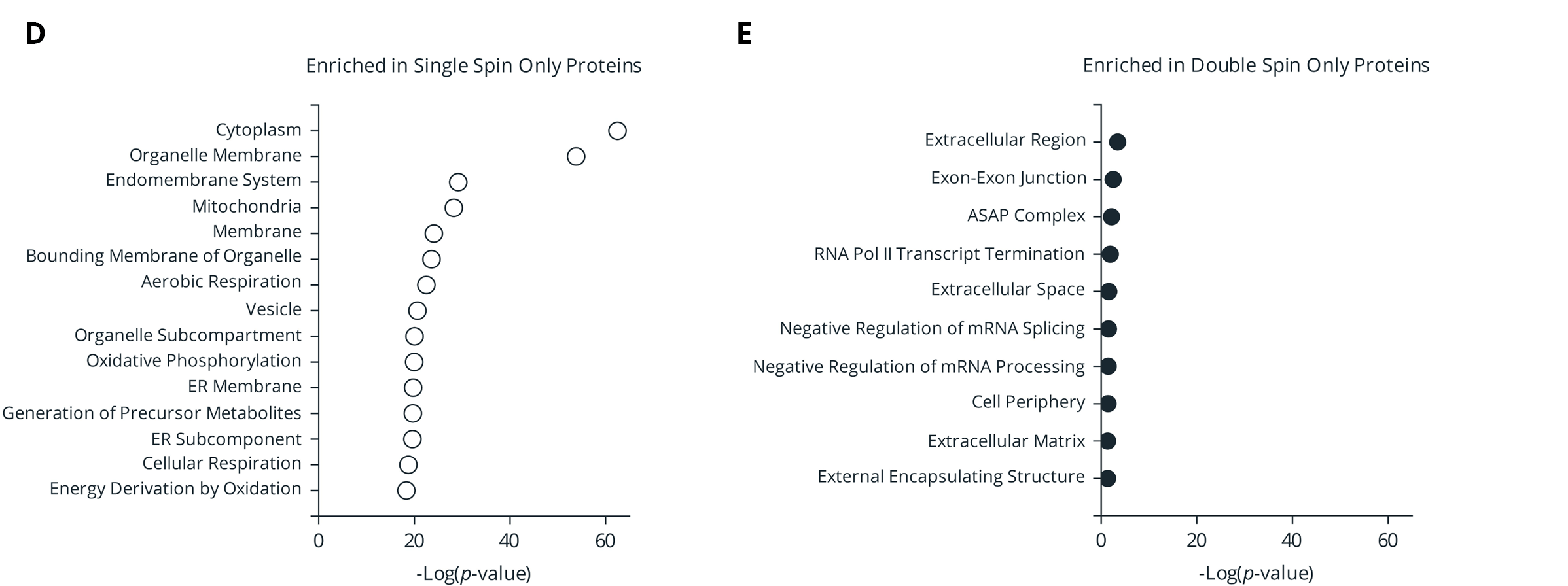
Figure 3. (A) Overlap of proteins identified in plasma samples isolated from single- or double-spin protocols. Proteins must be present in 70% of analyzed samples (Protein Plus, EDTA, or overall). (B-C) Mean Log2 fold change relative to draw time for proteins identified in only single- (B) or double- (C) spin samples. (D-E) Gene Ontology (GO) enrichment analysis of the proteins identified only in single-spin (D) or double-spin (E) samples. The enriched GO terms are displayed on the y-axis with the x-axis indicating the Log of the adjusted p-value for the significance of the enriched term.
Impact of prolonged exposure to extreme temperatures on the plasma proteome
Impact of prolonged exposure of whole blood to low temperatures on the plasma proteome
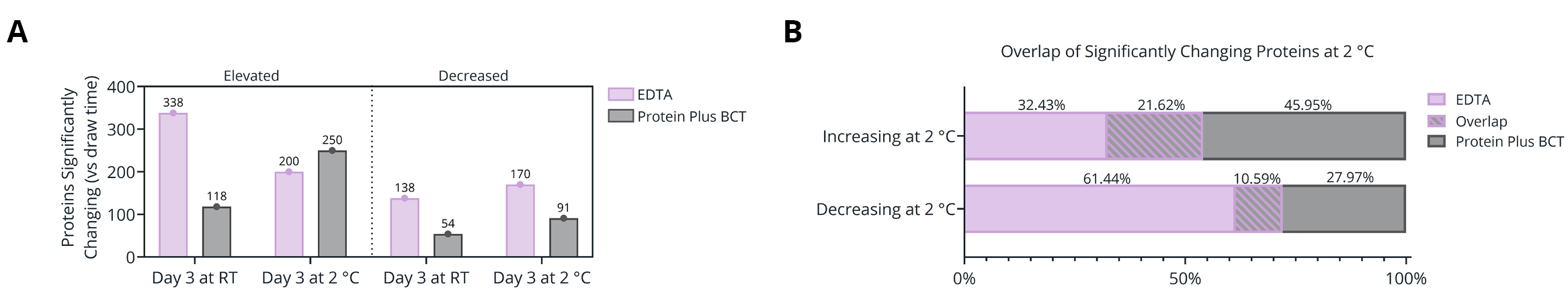

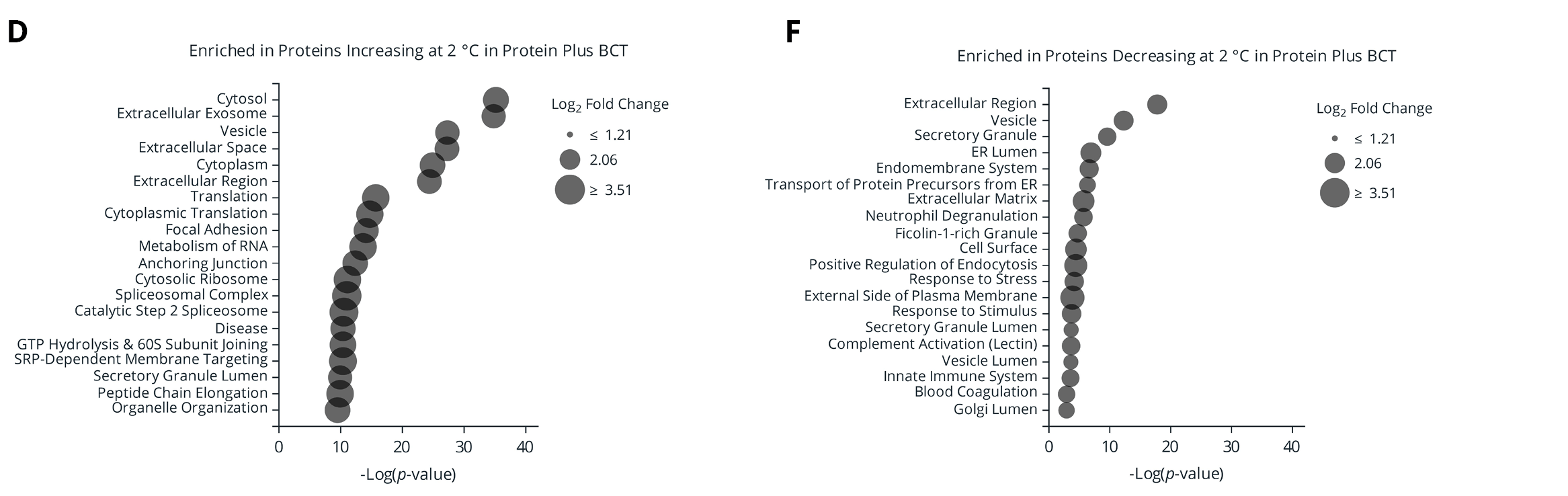
Figure 4. (A) Number of proteins significantly changing in abundance between plasma isolated from EDTA and Protein Plus BCT at draw time and after 3 days of whole blood storage at 2 °C or ambient temperature. (B) Protein identification overlap between tube types (EDTA and Protein Plus BCT) for proteins significantly changing in abundance relative to draw time levels. (C-F) Gene Ontology (GO) enrichment analysis of the proteins significantly increasing (C-D) or decreasing (E-F) in abundance following prolonged storage at 2 °C. The enriched GO terms are displayed on the y-axis with the x-axis indicating the Log of the adjusted p-value for the significance of the enriched term. Circle size corresponds to the mean Log2 fold change relative to draw time for all proteins associated with the given GO term.
Impact of prolonged exposure of whole blood to elevated temperatures on the plasma proteome
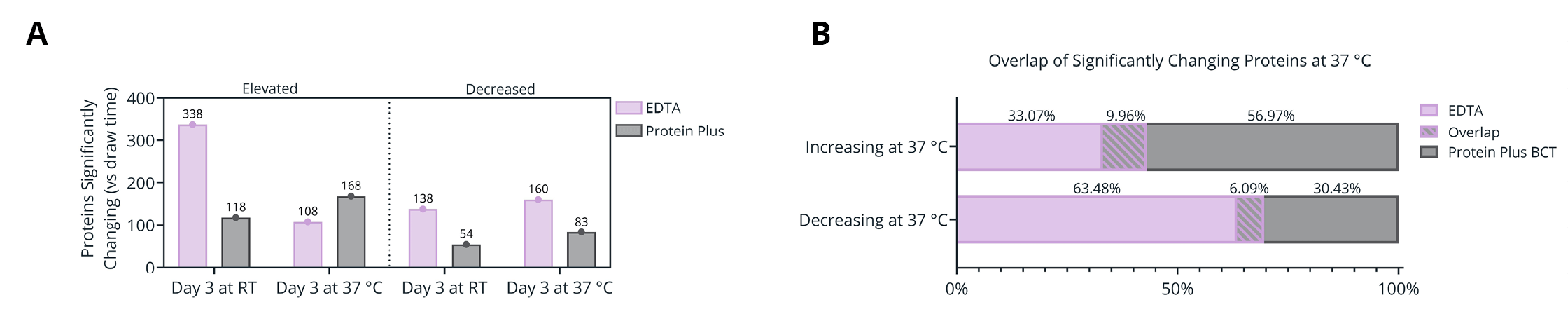
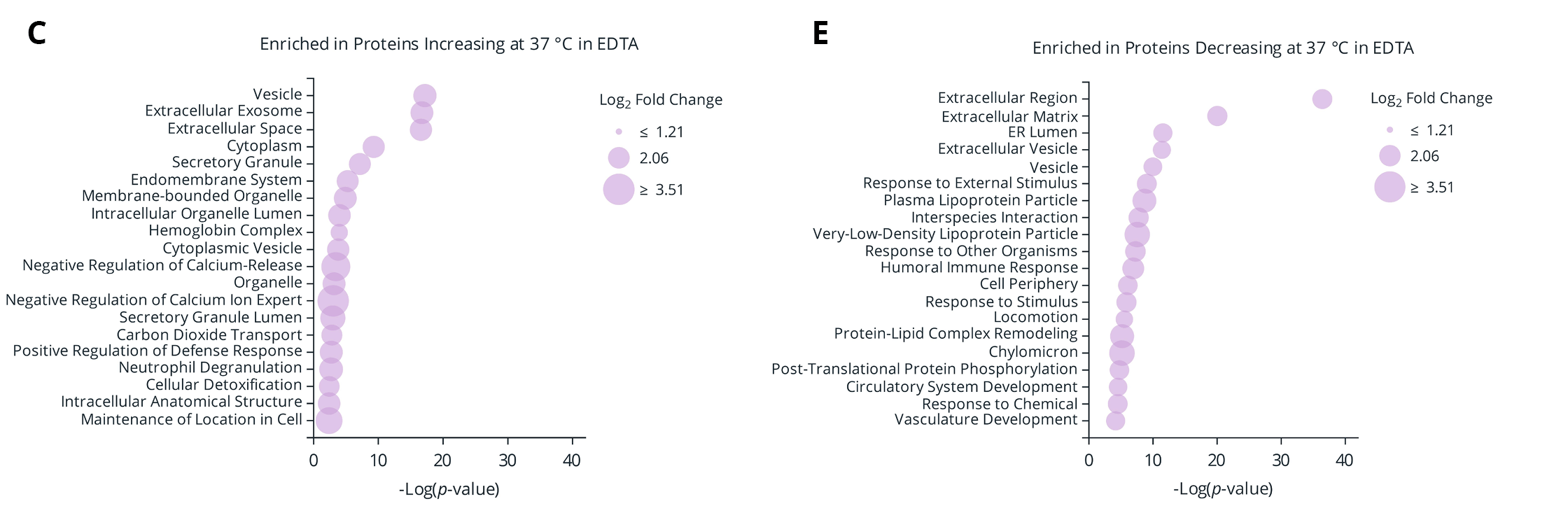
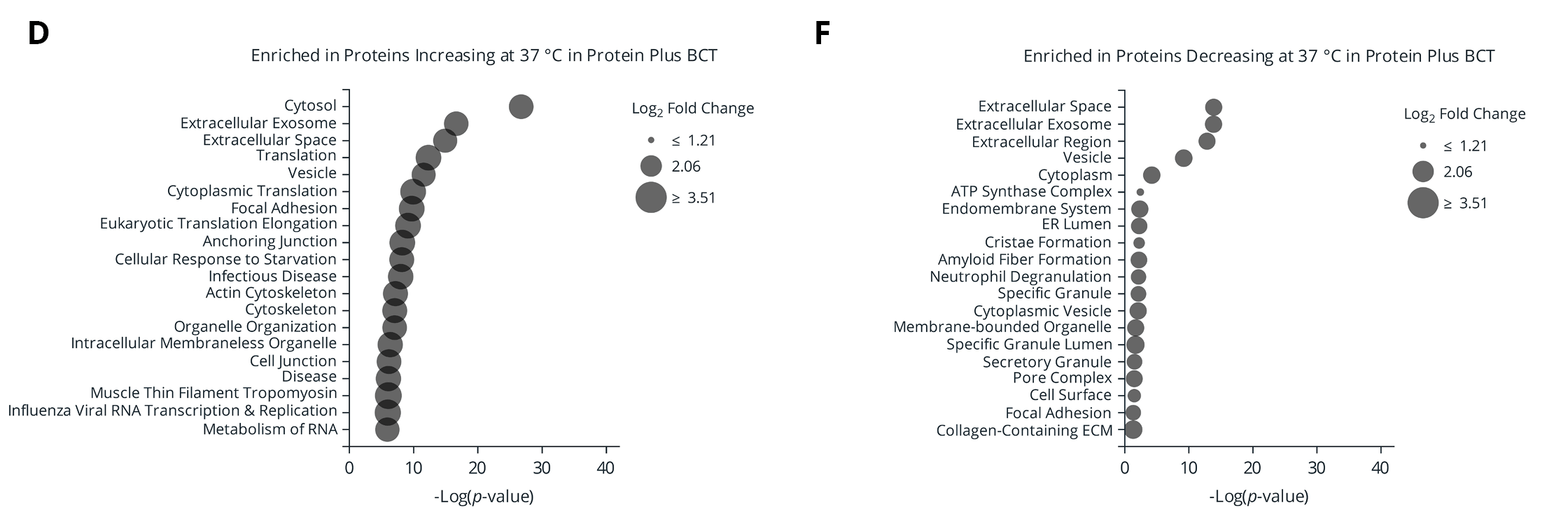
Figure 5. (A) Number of proteins significantly changing in abundance between plasma isolated from EDTA and Protein Plus BCT at draw time and after 3 days of whole blood storage at 37 °C or ambient temperature. (B) Protein identification overlap between tube types (EDTA and Protein Plus BCT) for proteins significantly changing in abundance relative to draw time levels. (C-F) Gene Ontology (GO) enrichment analysis of the proteins significantly increasing (C-D) or decreasing (E-F) in abundance following prolonged storage at 37 °C. The enriched GO terms are displayed on the y-axis with the x-axis indicating the Log of the adjusted p-value for the significance of the enriched term. Circle size to the mean Log2 fold change relative to draw time for all proteins associated with the given GO term.
Impact of prolonged exposure to extreme temperatures on blood cell-associated proteins
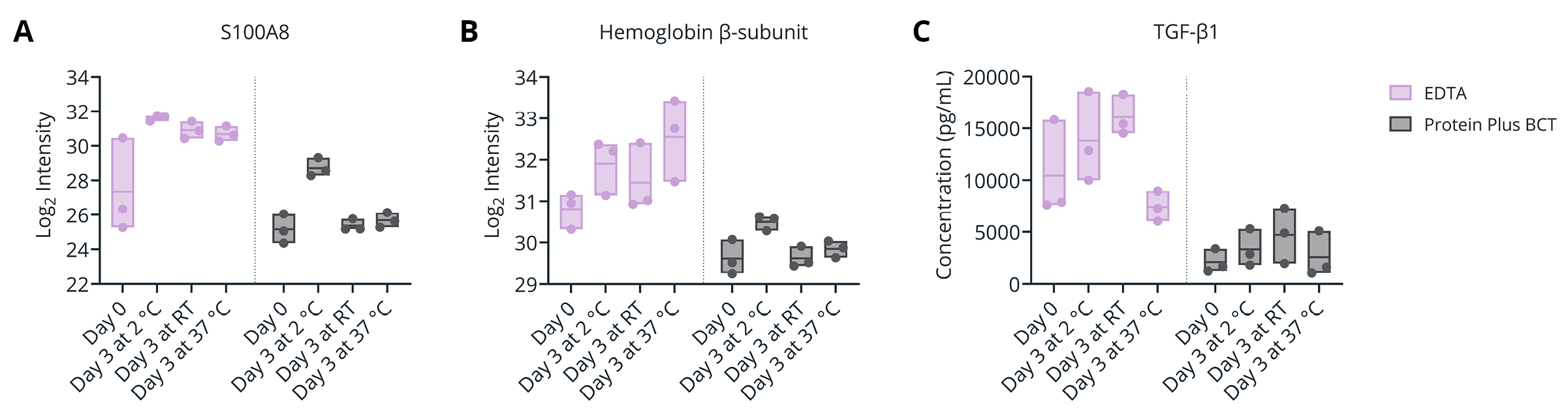
Figure 6. (A-B) Plasma levels (Log2 Intensity, by mass spectrometry) of blood cell-associated proteins S100A8 (A) and Hemoglobin β subunit (B) for samples isolated following prolonged (3 day) storage at 2 °C, room temperature (RT, 22 °C), or 37° C. (C) Plasma TGF-β1 concentration (pg/mL) as determined by immunoassay for samples isolated following prolonged (3 day) storage at 2 °C, room temperature (RT, 22 °C), or 37° C.
CONCLUSIONS
Here, we demonstrate the impact of alternative plasma isolations and whole blood storage temperature on plasma proteomic analysis. Plasma from samples collected into the conventional anticoagulant EDTA exhibited a dramatically higher number of proteins significantly changing in abundance following isolation with a single-spin protocol compared to a double-spin protocol. While Protein Plus BCT reduces the overall impact of single versus double spin, lower abundance levels of many proteins of interest in double-spun plasma remains. When whole blood experienced prolonged storage at low or elevated temperatures, proteome stabilization was impacted; however, at varied degrees due to the highly individual impact of temperature exposure on proteins, as not all proteins behave in the same way. Proteins significantly changing in abundance in plasma isolated from samples collected into EDTA following extreme temperature exposure were enriched for biological processes involving blood cell-associated proteins and extracellular vesicles. In contrast, samples collected into Protein Plus BCT better maintained blood
cell-associated proteins even with extreme temperature exposure. Taken together, these data indicate that the temperature at which whole blood is stored and how plasma is isolated can have a profound impact on the plasma proteome. Further, though Protein Plus BCT was designed to maintain the plasma proteome at room temperature storage and recommends double-spin plasma isolation, it may also be beneficial for alternative plasma handling and processing conditions.