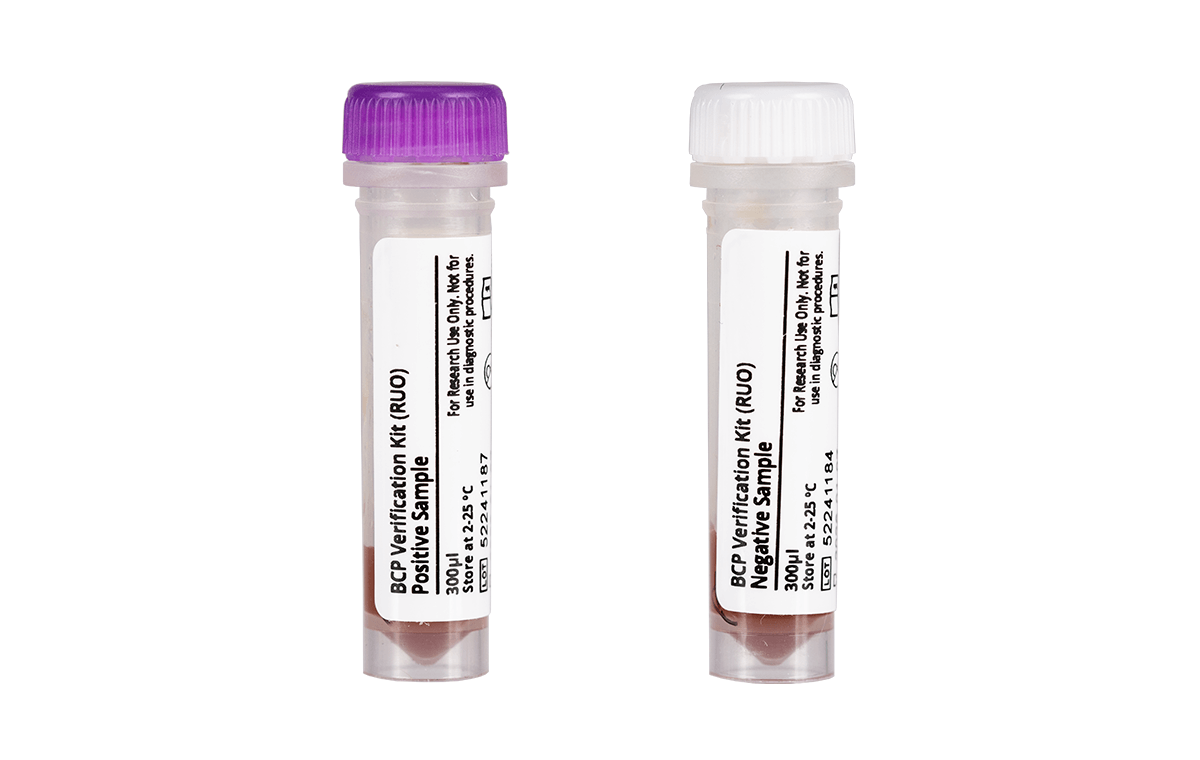
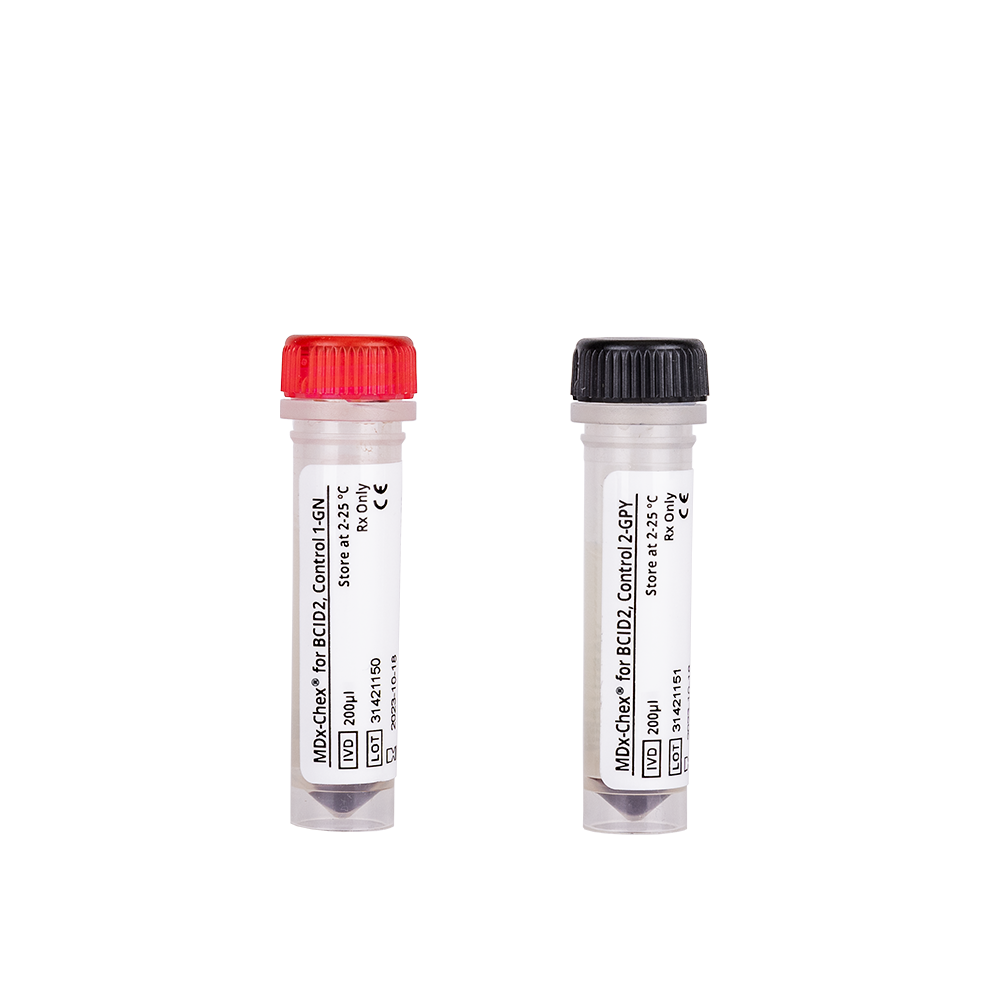
Blood Culture Yeast Verification Kit (RUO), Blood Culture GP Verification Kit (RUO) and Blood Culture GN Verification Kit (RUO)
Kits for the development of procedures for verification of molecular assay workflows
Blood Culture Yeast Verification Kit (RUO), Blood Culture GP Verification Kit (RUO) and Blood Culture GN Verification Kit (RUO) are designed to verify the performance of molecular testing processes that detect the presence of nucleic acids from various yeasts or bacteria. Supplied in single-use vials, these kits can also be used to train and evaluate operator proficiency.
For Research Use Only. Not for use in diagnostic procedures. We recommend that you confirm that kits used meet your laboratory’s sample-to-results system verification standards.
*See the Instructions For Use (IFU) for full target coverage.
Ordering Information
| Description | Item Number |
|---|---|
| Blood Culture Yeast Verification Kit (RUO) – 10 Tubes Positive Sample, 10 Tubes Negative Sample | 250092 |
| Blood Culture GP Verification Kit (RUO) – 10 Tubes Positive Sample, 10 Tube Negative Sample | 250093 |
| Blood Culture GN Verification Kit (RUO) – 10 Tubes Vial 1, 10 Tubes Vial 2 | 250094 |
Order Blood Culture Yeast Verification Kit (RUO), Blood Culture GP Verification Kit (RUO) and Blood Culture GN Verification Kit (RUO) today
New customers
Existing customers
International customers find a distributor
Resources
Instructions (IFU)
Assays
- Blood Culture GN Verification Kit (RUO) Assay Lot 5224 2026-01-07
- Blood Culture GP Verification Kit (RUO) Assay Lot 5224 2026-01-07
- Blood Culture Yeast Verification Kit (RUO) Assay Lot 5224 2026-01-07
- Blood Culture GN Verification Kit (RUO) Assay Lot 5252 2026-02-04
- Blood Culture GP Verification Kit (RUO) Assay Lot 5252 2026-02-04
- Blood Culture Yeast Verification Kit (RUO) Assay Lot 5252 2026-02-04
- Blood Culture GN Verification Kit (RUO) Assay Lot 5280 2026-03-04
- Blood Culture GP Verification Kit (RUO) Assay Lot 5280 2026-03-04
- Blood Culture Yeast Verification Kit (RUO) Assay Lot 5280 2026-03-04
- Blood Culture GN Verification Kit (RUO) Assay Lot 5308 2026-04-01
- Blood Culture GP Verification Kit (RUO) Assay Lot 5308 2026-04-01
- Blood Culture Yeast Verification Kit (RUO) Assay Lot 5308 2026-04-01
Certificates
- Blood Culture GN Verification Kit (RUO) CoC Lot 5224 2026-01-07
- Blood Culture GP Verification Kit (RUO) CoC Lot 5224 2026-01-07
- Blood Culture Yeast Verification Kit (RUO) CoC Lot 5224 2026-01-07
- Blood Culture GN Verification Kit (RUO) CoC Lot 5252 2026-02-04
- Blood Culture GP Verification Kit (RUO) CoC Lot 5252 2026-02-04
- Blood Culture Yeast Verification Kit (RUO) CoC Lot 5252 2026-02-04
- Blood Culture GN Verification Kit (RUO) CoC Lot 5280 2026-03-04
- Blood Culture GP Verification Kit (RUO) CoC Lot 5280 2026-03-04
- Blood Culture Yeast Verification Kit (RUO) CoC Lot 5280 2026-03-04
- Blood Culture GN Verification Kit (RUO) CoC Lot 5308 2026-04-01
- Blood Culture GP Verfication Kit (RUO) CoC Lot 5308 2026-04-01
- Blood Culture Yeast Verification Kit (RUO) CoC Lot 5308 2026-04-01
SDS
Looking for an older document? Click here for archived assays and certificates.
Molecular Products
With full-process controls and antibiotic resistance kits, Streck has the products to help you streamline your molecular diagnostics testing, aid antibiotic stewardship and ensure positive patient outcomes.
The latest from the blog


Streck releases improved ARM-D Kit, OXA