Molecular Diagnostic Controls
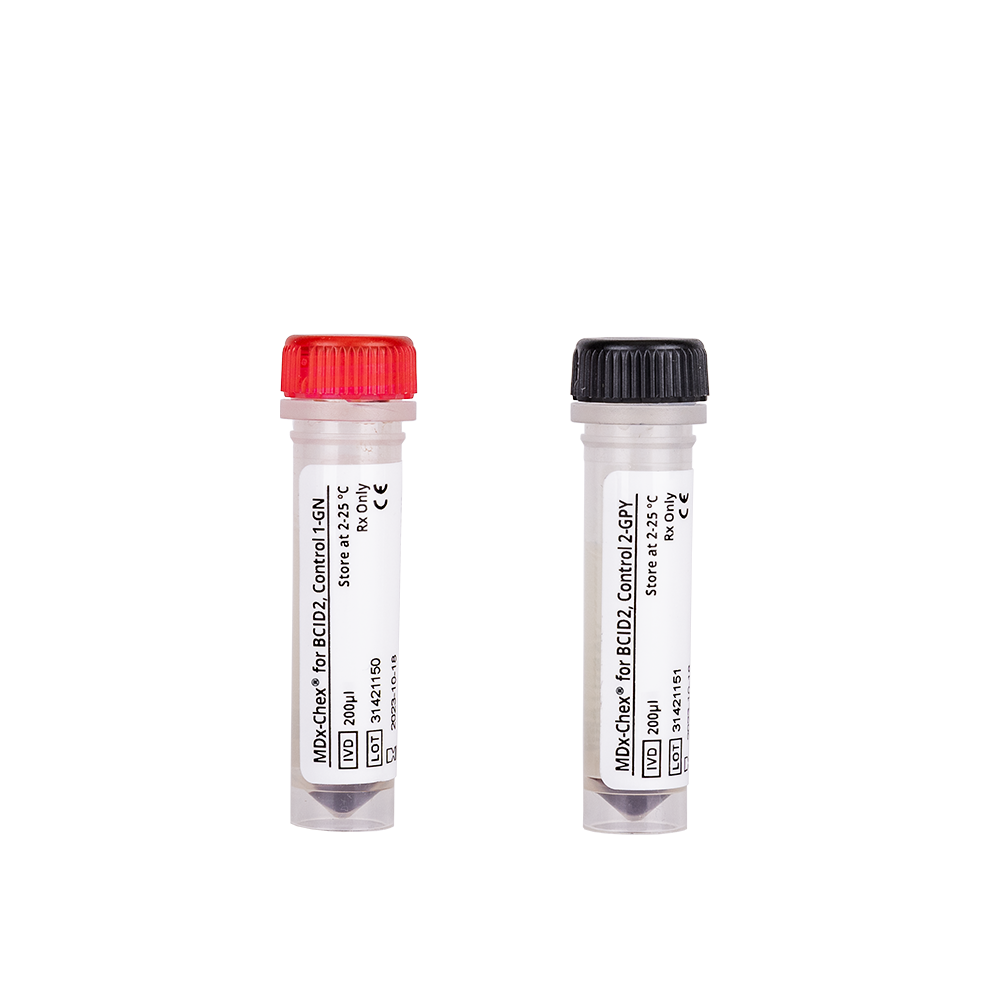
Our line of MDx-Chex® molecular diagnostic controls provides an all-in-one solution for verifying the performance of molecular assays that identify various yeasts or bacteria in blood cultures. Comprehensive and easy-to-use, MDx-Chex controls evaluate the entire assay process, from cell lysis, DNA extraction and purification to nucleic acid detection and analysis.
MDx-Chex controls are the best choice for your molecular assay workflow validation:
- Ensure your instruments, test kits and lab technicians are accurate
- Comply with the newest recommendations from CAP, CSLI and ASM for external controls that assess all aspects of the testing process
- Perform consistently from lot-to-lot
Need to verify your molecular assay workflow?
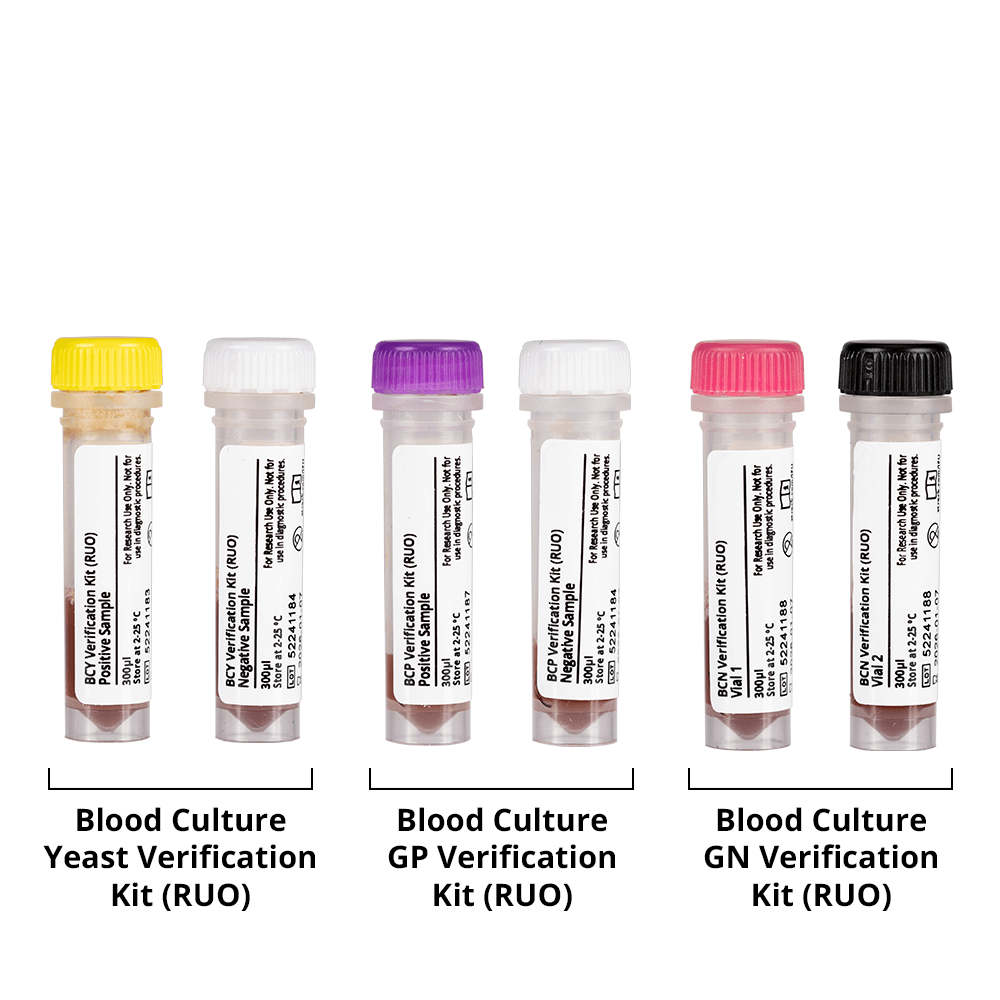
Our verification kits can be used to develop and perform protocols for the verification of molecular testing processes that detect the presence of nucleic acids from various yeasts or bacteria.
AMP 2025 – Behind Every Sample: A Sepsis Survivor’s Story and Role of the Lab
Hear a survivor share her journey from early symptoms to septic shock, and join Chris Connelly, Ph.D., Director of Business Segment – Molecular at Streck, as he leads a discussion on how labs and clinicians can work together to deliver faster, more reliable results.
Keep sepsis testing on target with MDx-Chex controls
Watch this video to learn how MDx-Chex controls comprehensively evaluate the performance of your BIOFIRE BCID2 and VERIGENE BC-GP and BC-GN sepsis tests.
A step-by-step instruction for using MDx-Chex for BCID2 with the BIOFIRE FilmArray® 2.0 and Torch Systems.
The latest from the blog


Understanding proteoforms and their impact