Fecal transplants bugged by C. diff
Topics Featured
The Centers for Disease Control and Prevention has declared November to be Clostridioides difficile Awareness Month. The CDC wants to reduce instances of Clostridioides difficile (C. diff) infection by 30% by 2020.
C. diff has been in the headlines recently. In June, the FDA issued a safety alert when one patient died and another suffered an invasive infection after fecal microbiota transplantation (FMT) treatment. FMT is an emerging therapy for people who develop recurrent or resistant C. diff infection. C. diff is a bacterium that causes diarrhea and colitis (inflammation of the colon).
The affected patients had received FMTs from the same donor. The problems were caused by extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli.
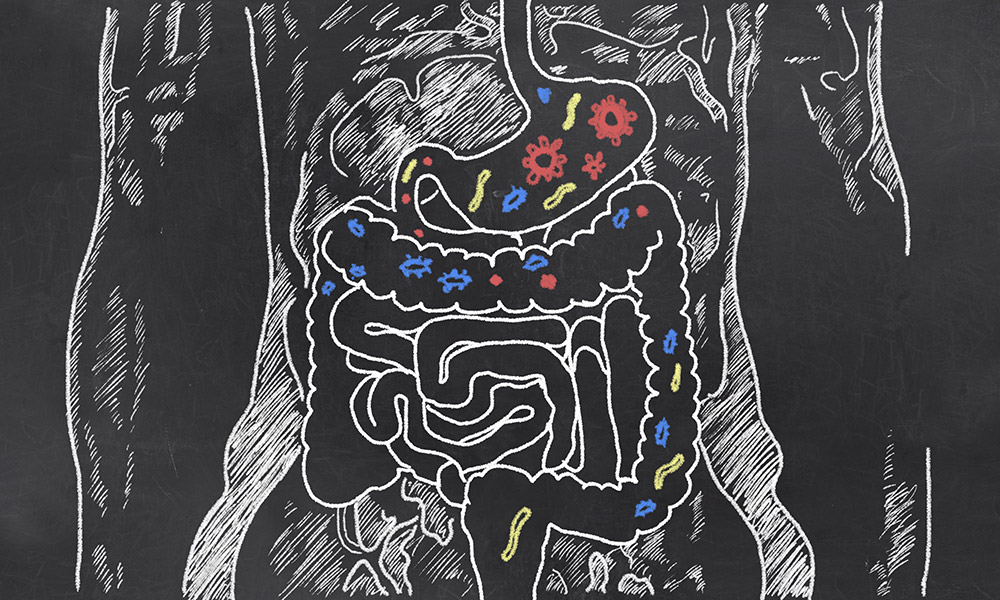
In FMT, the intestinal microbes from a healthy donor are administered to a recipient with the intent of modifying that person’s intestinal microbiome. The microbiome is a group of trillions of microbes that include bacteria, fungi, parasites and viruses. In a healthy person, these “bugs” coexist peacefully, with the largest numbers found in the small and large intestines.
But if there is a disturbance in that balance ‒ brought on by infectious illnesses, certain diets, or the prolonged use of antibiotics or other bacteria-destroying medications ‒ the body may become more susceptible to disease.
You don’t want to introduce bugs that are resistant to antibiotics to an already sick person. The New England Journal of Medicine recently published a report that suggested FMT donors should be screened for resistance infections before their fecal material is transplanted to immune compromised patients, such as cancer patients. Additionally, the FDA now recommends that doctors performing fecal transplants follow some new safety measures, including screening donors to see if they are at risk of carrying multidrug-resistant organisms (MDROs), testing donor stool for MDROs and clearly outlining potential risks to patients considering fecal transplants.
In 2017, there were an estimated 223,900 cases of C. diff in hospitalized patients and 12,800 deaths in the United States, according to the CDC.
The CDC is also working to implement antibiotic stewardship programs across all healthcare settings, as research shows that the spike in C. difficile may be linked to antibiotic overuse. According to the CDC’S 2019 Antibiotic Resistance Threats Report, there was an estimated 223,900 cases of C. diff in hospitalized patients and 12,800 deaths in the United States.
One way hospital infection control staff can check to see which resistance mechanisms are present in their local community or health system is to use an antibiotic resistance surveillance method, such as Streck ARM-D® Kits. These kits allow lab personnel to detect the most clinically important β-lactamases for current and emerging threats.
For information about our line of antibiotic resistance detection kits and to browse webinars, talks and posters related to antimicrobial resistance, visit our resources page.
Streck ARM-D Kits are for Research Use Only. Not for use in diagnostic procedures.
New antibiotic fights antimicrobial resistant bacteria